| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
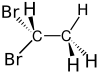
1,1-Dibromoethane[1] | |||
| Other names
Ethylidene bromide, ethylidene dibromide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.008.351 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H4Br2 | |||
| Molar mass | 187.862 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Melting point | −63.0 °C; −81.3 °F; 210.2 K | ||
| Boiling point | 108.1 °C; 226.5 °F; 381.2 K | ||
| 3.4 g/L (25 °C) | |||
| Solubility | soluble in ether, ethanol, acetone,and benzene slight soluble chloroform | ||
| log P | 1.9 (estimated) | ||
Refractive index (nD)
|
1.51277 (at 20 °C) | ||
| Hazards | |||
| GHS labelling: | |||
| Danger | |||
| H301, H311, H315, H319, H331 | |||
| P261, P264, P270, P271, P280, P311, P312, P321, P322, P330, P361, P362, P363, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | > 93 °C (199 °F; 366 K) | ||
| Safety data sheet (SDS) | fishersci.com | ||
| Related compounds | |||
Related alkanes
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
1,1-Dibromoethane is a clear, slightly brown, flammable chemical compound.[3] It is classified as the organobromine compound, and has the chemical formula C2H4Br2[4] and it is a position isomer of 1,2-dibromoethane. It is commonly seen in industrial chemistry, where it is used as a fuel additive.[5] It is also used as a grain and soil fumigant for insect control.[6]
- ^ "Ethylidene dibromide - Compound Summary". PubChem Compound. US: National Center for Biotechnology Information. 27 March 2005. Identification. Retrieved 19 June 2012.
- ^ "1,1-Dirbomoethane". National Center for Biotechnology Information. Retrieved 31 May 2017.
- ^ "MSDS". Fisher Scientific, Inc. Retrieved 13 June 2012.
- ^ "Dibromoethane". ChemSpider. Retrieved 13 June 2012.
- ^ "1,1-dibromoethane". PubChem. Retrieved 9 June 2017.
- ^ Larranaga, Michael (10 March 2016). Hawley's Condensed Chemical Dictionary. John Wiley & Sons. ISBN 9781118135150. Retrieved 9 June 2017.