| |||
| |||
| Names | |||
|---|---|---|---|
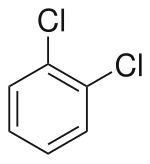
| Preferred IUPAC name
1,2-Dichlorobenzene | |||
| Other names
ortho-Dichlorobenzene, o-Dichlorobenzene, odcb, o-Dichlorobenzol
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.206 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H4Cl2 | |||
| Molar mass | 147.01 g/mol | ||
| Appearance | colourless liquid | ||
| Odor | Naphthalene-like | ||
| Density | 1.30 g/cm3 | ||
| Melting point | −17.03 °C (1.35 °F; 256.12 K) | ||
| Boiling point | 180.19 °C (356.34 °F; 453.34 K)[3] | ||
| 0.01%[2] | |||
| Vapor pressure | 1 mmHg (20°C) | ||
| -84.26·10−6 cm3/mol | |||
Refractive index (nD)
|
1.54920 | ||
| Viscosity | 1.0656 (20 °C) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Ingestion hazards
|
Mildly toxic | ||
Inhalation hazards
|
Causes respiratory tract irritation | ||
Eye hazards
|
Causes eye irritation | ||
Skin hazards
|
Causes skin irritation | ||
| Flash point | 66 °C (151 °F; 339 K) | ||
| Explosive limits | 2.2%-9.2%[2] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
500 mg/kg (oral, rat, rabbit) 200 mg/kg (oral, guinea pig) 436 mg/kg (oral, mouse)[4] | ||
LCLo (lowest published)
|
1000 ppm (guinea pig, 20 hr) 800 ppm (guinea pig, 24 hr) 821 ppm (rat, 7 hr)[4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
C 50 ppm (300 mg/m3)[2] | ||
REL (Recommended)
|
C 50 ppm (300 mg/m3)[2] | ||
IDLH (Immediate danger)
|
200 ppm[2] | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related compounds
|
1,2-Difluorobenzene 1,2-Dibromobenzene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
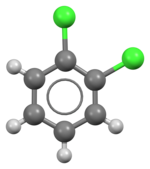
1,2-Dichlorobenzene, or orthodichlorobenzene (ODCB), is an aryl chloride and isomer of dichlorobenzene with the formula C6H4Cl2. This colourless liquid is poorly soluble in water but miscible with most organic solvents. It is a derivative of benzene, consisting of two adjacent chlorine atoms.
It is mainly used as a precursor chemical in the synthesis of agrochemicals, as a preferred solvent for dissolving and working with fullerenes, as an insecticide, and in softening and removing carbon-based contamination on metal surfaces.
- ^ Merck Index, 11th Edition, 3044
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0189". National Institute for Occupational Safety and Health (NIOSH).
- ^ Roháč, Vladislav; Růžička, Vlastimil; Růžička, Květoslav; Aim, Karel (September 1998). "Measurements of Saturated Vapor Pressure above the Liquid Phase for Isomeric Dichlorobenzenes and 1,2,4-Trichlorobenzene". Journal of Chemical & Engineering Data. 43 (5): 770–775. doi:10.1021/je9701442. ISSN 0021-9568.
- ^ a b "o-Dichlorobenzene". National Institute for Occupational Safety and Health (NIOSH). 4 December 2014. Retrieved 6 March 2015.