| |

| |
| Names | |
|---|---|
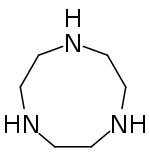
| Preferred IUPAC name
1,4,7-Triazonane | |
| Identifiers | |
3D model (JSmol)
|
|
| 773877 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.164.887 |
| EC Number |
|
| 2614 | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H15N3 | |
| Molar mass | 129.2046 g/mol |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1,4,7-Triazacyclononane, known as "TACN" which is pronounced "tack-en," is an aza-crown ether with the formula (C2H4NH)3.[1] TACN is derived, formally speaking, from cyclononane by replacing three equidistant CH2 groups with NH groups. TACN is one of the oligomers derived from aziridine, C2H4NH. Other members of the series include piperazine, C4H8(NH)2, and the cyclic tetramer 1,4,7,10-tetraazacyclododecane.
- ^ Chaudhuri, P.; Wieghardt, K. (1987). "The Chemistry of 1,4,7-Triazacyclononane and Related Tridentate Macrocyclic Compounds". In Lippard, Stephen J. (ed.). Progress in Inorganic Chemistry. Vol. 35. Hoboken, NJ, USA: John Wiley & Sons, Inc. pp. 329–436. doi:10.1002/9780470166369.ch4. ISBN 9780470166369.