 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
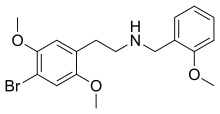
| Formula | C18H22BrNO3 |
| Molar mass | 380.282 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
25B-NBOMe (NBOMe-2C-B, Cimbi-36, Nova, BOM 2-CB) is a derivative of the phenethylamine psychedelic 2C-B, discovered in 2004 by Ralf Heim at the Free University of Berlin. It acts as a potent full agonist for the 5HT2A receptor.[3][4][5] Duration of effects lasts about 3–10 hours,[6] although the parent compound is rapidly cleared from the blood when used in the radiolabeled form in tracer doses.[7] Recently, Custodio et al. (2019) evaluated the potential involvement of dysregulated dopaminergic system, neuroadaptation, and brain wave changes which may contribute to the rewarding and reinforcing properties of 25B-NBOMe in rodents.[8]
The carbon-11 labeled version of this compound ([11C]Cimbi-36) was synthesized and validated as a radioactive tracer for positron emission tomography (PET) in Copenhagen.[9][10][11] As a 5-HT2A receptor agonist PET radioligand, [11C]Cimbi-36 was hypothesized to provide a more functional marker of these receptors. Also, [11C]Cimbi-36 is investigated as a potential marker of serotonin release and thus could serve as an indicator of serotonin levels in vivo. [11C]Cimbi-36 is now undergoing clinical trials as a PET-ligand in humans.[12][13][14]
- ^ Anvisa (July 24, 2023). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published July 25, 2023). Archived from the original on August 27, 2023. Retrieved August 27, 2023.
- ^ "Substance Details 25B-NBOMe". Retrieved January 22, 2024.
- ^ Silva M (2009). Theoretical study of the interaction of agonists with the 5-HT2A receptor (Ph.D. thesis). Universität Regensburg.
- ^ Silva ME, Heim R, Strasser A, Elz S, Dove S (January 2011). "Theoretical studies on the interaction of partial agonists with the 5-HT2A receptor". Journal of Computer-Aided Molecular Design. 25 (1): 51–66. Bibcode:2011JCAMD..25...51S. CiteSeerX 10.1.1.688.2670. doi:10.1007/s10822-010-9400-2. PMID 21088982. S2CID 3103050.
- ^ Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (March 2014). "Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists". ACS Chemical Neuroscience. 5 (3): 243–9. doi:10.1021/cn400216u. PMC 3963123. PMID 24397362.
- ^ Francesco SB, Ornella C, Gabriella A, Giuseppe V, Rita S, Flaminia BP, Eduardo C, Pierluigi S, Giovanni M, Guiseppe B, Fabrizio S (July 3, 2014). "25C-NBOMe: preliminary data on pharmacology, psychoactive effects, and toxicity of a new potent and dangerous hallucinogenic drug". BioMed Research International. 2014: 734749. doi:10.1155/2014/734749. PMC 4106087. PMID 25105138.
- ^ Ettrup A, da Cunha-Bang S, McMahon B, Lehel S, Dyssegaard A, Skibsted AW, et al. (July 2014). "Serotonin 2A receptor agonist binding in the human brain with [¹¹C]Cimbi-36". Journal of Cerebral Blood Flow and Metabolism. 34 (7): 1188–96. doi:10.1038/jcbfm.2014.68. PMC 4083382. PMID 24780897.
- ^ Custodio RJ, Sayson LV, Botanas CJ, Abiero A, You KY, Kim M, et al. (November 2020). "25B-NBOMe, a novel N-2-methoxybenzyl-phenethylamine (NBOMe) derivative, may induce rewarding and reinforcing effects via a dopaminergic mechanism: Evidence of abuse potential". Addiction Biology. 25 (6): e12850. doi:10.1111/adb.12850. PMID 31749223. S2CID 208217863.
- ^ Hansen M (December 16, 2010). Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain (Ph.D. thesis). University of Copenhagen. doi:10.13140/RG.2.2.33671.14245.
- ^ Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, Lehel S, Herth MM, Madsen J, et al. (April 2011). "Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers". European Journal of Nuclear Medicine and Molecular Imaging. 38 (4): 681–93. doi:10.1007/s00259-010-1686-8. PMID 21174090. S2CID 12467684.
- ^ Ettrup A, Holm S, Hansen M, Wasim M, Santini MA, Palner M, et al. (August 2013). "Preclinical safety assessment of the 5-HT2A receptor agonist PET radioligand [ 11C]Cimbi-36". Molecular Imaging and Biology. 15 (4): 376–383. doi:10.1007/s11307-012-0609-4. PMID 23306971. S2CID 1474367.
- ^ "From molecule to man: The full CIMBI-36 story" (PDF). cimbi.dk. Retrieved January 10, 2014.
- ^ "Imanova announces the launch of a new imaging biomarker to investigate the serotonin system in psychiatric illness". imanova.co.uk. Archived from the original on April 9, 2015. Retrieved April 9, 2015.
- ^ Madsen MK, Fisher PM, Burmester D, Dyssegaard A, Stenbæk DS, Kristiansen S, et al. (June 2019). "Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels". Neuropsychopharmacology. 44 (7): 1328–1334. doi:10.1038/s41386-019-0324-9. PMC 6785028. PMID 30685771.