| |
| Names | |
|---|---|
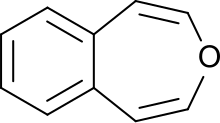
| Preferred IUPAC name
3-Benzoxepine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H8O | |
| Molar mass | 144.173 g·mol−1 |
| Appearance | Yellow solid[1] |
| Melting point | 84 (83–84 °C;[3] 84 °C[1]) |
| Solubility | soluble in apolar solvents (diethyl ether, benzene, tetrachloromethane)[2] and alcohols (methanol)[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
3-Benzoxepin is an annulated ring system with an aromatic benzene ring and a non-aromatic, unsaturated, oxygen-containing seven-membered heterocyclic oxepin. The first synthesis was described by Karl Dimroth and coworkers in 1961.[1] It is one of the three isomers of the benzoxepins.
- ^ a b c Dimroth, K.; Pohl, G. (1961). "3-Benzoxepin". Angew. Chem. 73 (12): 436. Bibcode:1961AngCh..73..436D. doi:10.1002/ange.19610731215.
- ^ Cite error: The named reference
Rosowskywas invoked but never defined (see the help page). - ^ a b Dimroth, K.; Pohl, G.; Follmann, H. (1966). "Die Synthese von Derivaten des 3-Oxepins und des Furans durch eine zweifache Wittig-Reaktion". Chem. Ber. (in German). 99 (2): 634–641. doi:10.1002/cber.19660990238.