 | |
 | |
| Clinical data | |
|---|---|
| Trade names | 3FPPA |
| Addiction liability | moderate[1] |
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Onset of action | 20 - 60 minutes |
| Elimination half-life | 90 minutes |
| Duration of action | 2 - 3 hours "3-FA". Psychonautwiki.[unreliable medical source?] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
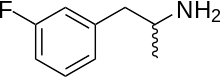
| Formula | C9H12FN |
| Molar mass | 153.200 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.0 [2] g/cm3 |
| Boiling point | 208.2[2] °C (406.8 °F) |
| |
| |
| | |
3-Fluoroamphetamine (3-FA; PAL-353) is a stimulant drug from the amphetamine family which acts as a monoamine releaser with similar potency to methamphetamine but more selectivity for dopamine and norepinephrine release over serotonin.[3] It is self-administered by mice to a similar extent to related drugs such as 4-fluoroamphetamine and 3-methylamphetamine.[4]
3-Fluoroamphetamine often found its use as a designer drug in several studies to mimic the effects of illegal amphetamines.[5] It has also appeared on the drug market for recreational use as an amphetamine alternative, its has been reported in January 2009 to the European Early Warning System by Belgium. Little is known about the exact history of this compound.[6]
- ^ Cite error: The named reference
Puri_2017was invoked but never defined (see the help page). - ^ a b "3-Fluoroamphetamine | C9H12FN". Chemspider. 2022.
- ^ Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB (February 2007). "Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate "agonist" medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys". The Journal of Pharmacology and Experimental Therapeutics. 320 (2): 627–36. doi:10.1124/jpet.106.107383. PMID 17071819. S2CID 8326027.
- ^ Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL (May 2005). "Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs". The Journal of Pharmacology and Experimental Therapeutics. 313 (2): 848–54. doi:10.1124/jpet.104.080101. PMID 15677348. S2CID 12135483.
- ^ Seibert E, Mader E, Schmid MG (November 2021). "A simple and isocratic protein-based high performance liquid chromatography method for the enantioseparation of amphetamine derivatives". Journal of Chromatography Open. 1: 100013. doi:10.1016/j.jcoa.2021.100013. ISSN 2772-3917.
- ^ "GLOBAL SMART UPDATE 2009 Volume 2" (PDF). www.unodc.org. United Nations Office on Drugs and Crime. October 1, 2009.