 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Paser, Granupas, others |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 50–60% |
| Metabolism | liver |
| Excretion | kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.557 |
| Chemical and physical data | |
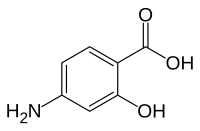
| Formula | C7H7NO3 |
| Molar mass | 153.137 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 150.5 °C (302.9 °F) |
| |
| |
| (verify) | |
4-Aminosalicylic acid, also known as para-aminosalicylic acid (PAS) and sold under the brand name Paser among others, is an antibiotic primarily used to treat tuberculosis.[2] Specifically it is used to treat active drug resistant tuberculosis together with other antituberculosis medications.[3] It has also been used as a second line agent to sulfasalazine in people with inflammatory bowel disease such as ulcerative colitis and Crohn's disease.[3] It is typically taken by mouth.[3]
Common side effects include nausea, abdominal pain, and diarrhea.[3] Other side effects may include liver inflammation and allergic reactions.[3] It is not recommended in people with end stage kidney disease.[3] While there does not appear to be harm with use during pregnancy it has not been well studied in this population.[3] 4-Aminosalicylic acid is believed to work by blocking the ability of bacteria to make folic acid.[3]
4-Aminosalicylic acid was first made in 1902, and came into medical use in 1943.[4] It is on the World Health Organization's List of Essential Medicines.[5]
- ^ Cite error: The named reference
Granupas EPARwas invoked but never defined (see the help page). - ^ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 140. hdl:10665/44053. ISBN 9789241547659.
- ^ a b c d e f g h "Aminosalicylic Acid". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- ^ Donald PR, Diacon AH (September 2015). "Para-aminosalicylic acid: the return of an old friend". The Lancet. Infectious Diseases. 15 (9): 1091–1099. doi:10.1016/s1473-3099(15)00263-7. PMID 26277036.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.