 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
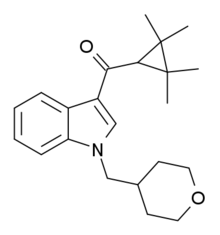
| Formula | C22H29NO2 |
| Molar mass | 339.479 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
A-834,735 is a drug developed by Abbott Laboratories that acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors, with a Ki of 12 nM at CB1 and 0.21 nM at CB2. Replacing the aromatic 3-benzoyl or 3-naphthoyl group found in most indole derived cannabinoids with the 3-tetramethylcyclopropylmethanone group of A-834,735 and related compounds imparts significant selectivity for CB2, with most compounds from this group found to be highly selective CB2 agonists with little affinity for CB1. However, low nanomolar CB1 binding affinity is retained with certain heterocyclic 1-position substituents such as (N-methylpiperidin-2-yl)methyl (cf. AM-1220, AM-1248), or the (tetrahydropyran-4-yl)methyl substituent of A-834,735, resulting in compounds that still show significant affinity and efficacy at both receptors despite being CB2 selective overall.[1][2][3][4][5]
- ^ Dart M, Frost J, Tietje K, Daza A, Grayson G, Fan Y, et al. (2006). "1-Alkyl-3-keto-indoles: identification and in vitro characterization of a series of potent cannabinoid ligands". 2006 Symposium on the Cannabinoids. Burlington, VT: International Cannabinoid Research Society.
- ^ Poso A, Huffman JW (January 2008). "Targeting the cannabinoid CB2 receptor: modelling and structural determinants of CB2 selective ligands". British Journal of Pharmacology. 153 (2): 335–46. doi:10.1038/sj.bjp.0707567. PMC 2219524. PMID 17982473.
- ^ Chin CL, Tovcimak AE, Hradil VP, Seifert TR, Hollingsworth PR, Chandran P, et al. (January 2008). "Differential effects of cannabinoid receptor agonists on regional brain activity using pharmacological MRI". British Journal of Pharmacology. 153 (2): 367–79. doi:10.1038/sj.bjp.0707506. PMC 2219521. PMID 17965748.
- ^ Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, et al. (March 2008). "Indol-3-yl-tetramethylcyclopropyl ketones: effects of indole ring substitution on CB2 cannabinoid receptor activity". Journal of Medicinal Chemistry. 51 (6): 1904–12. doi:10.1021/jm7011613. PMID 18311894.
- ^ Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, et al. (January 2010). "Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity". Journal of Medicinal Chemistry. 53 (1): 295–315. doi:10.1021/jm901214q. PMID 19921781.