
In organic chemistry, the acenes or polyacenes are a class of organic compounds and polycyclic aromatic hydrocarbons made up of benzene (C6H6) rings which have been linearly fused.[1][2] They follow the general molecular formula C4n+2H2n+4.
The larger representatives have potential interest in optoelectronic applications and are actively researched in chemistry and electrical engineering. Pentacene has been incorporated into organic field-effect transistors, reaching charge carrier mobilities as high as 5 cm2/Vs.
The first 5 unsubstituted members are listed in the following table:
| Name | Number of rings | Molecular formula | Structural formula |
|---|---|---|---|
| Anthracene | 3 | C14H10 | 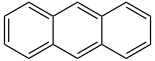
|
| Tetracene | 4 | C18H12 | 
|
| Pentacene | 5 | C22H14 | 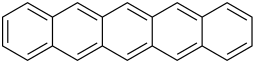
|
| Hexacene | 6 | C26H16 | 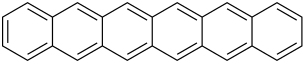
|
| Heptacene | 7 | C30H18 | 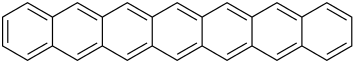
|
Hexacene is not stable in air, and dimerises upon isolation. Heptacene (and larger acenes) is very reactive and has only been isolated in a matrix. However, bis(trialkylsilylethynylated) versions of heptacene have been isolated as crystalline solids.[3]