| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Prop-2-enamide[2] | |||
| Other names
Acrylamide
Acrylic amide[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.067 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H5NO | |||
| Molar mass | 71.079 g·mol−1 | ||
| Appearance | white crystalline solid, no odor[1] | ||
| Density | 1.322 g/cm3 | ||
| Melting point | 84.5 °C (184.1 °F; 357.6 K) | ||
| Boiling point | None (polymerization); decomposes at 175-300°C[1] | ||
| 390 g/L (25 °C)[3] | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
potential occupational carcinogen[1] | ||
| GHS labelling: | |||
  [4] [4]
| |||
| H301, H312, H315, H317, H319, H332, H340, H350, H361, H372[4] | |||
| P201, P280, P301+P310, P305+P351+P338, P308+P313[4] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 138 °C (280 °F; 411 K) | ||
| 424 °C (795 °F; 697 K) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
100-200 mg/kg (mammal, oral) 107 mg/kg (mouse, oral) 150 mg/kg (rabbit, oral) 150 mg/kg (guinea pig, oral) 124 mg/kg (rat, oral)[5] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 0.3 mg/m3 [skin][1] | ||
REL (Recommended)
|
Ca TWA 0.03 mg/m3 [skin][1] | ||
IDLH (Immediate danger)
|
60 mg/m3[1] | ||
| Safety data sheet (SDS) | ICSC 0091 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
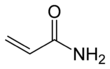
Acrylamide (or acrylic amide) is an organic compound with the chemical formula CH2=CHC(O)NH2. It is a white odorless solid, soluble in water and several organic solvents. From the chemistry perspective, acrylamide is a vinyl-substituted primary amide (CONH2). It is produced industrially mainly as a precursor to polyacrylamides, which find many uses as water-soluble thickeners and flocculation agents.[6]
Acrylamide forms in burnt areas of food, particularly starchy foods like potatoes, when cooked with high heat, above 120 °C (248 °F).[7] Despite health scares following its discovery in 2002, and its classification as a probable carcinogen, acrylamide from diet is thought unlikely to cause cancer in humans; Cancer Research UK categorized the idea that eating burnt food causes cancer as a "myth".[8][9]
- ^ a b c d e f g NIOSH Pocket Guide to Chemical Hazards. "#0012". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 842. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ "Human Metabolome Database: Showing metabocard for Acrylamide (HMDB0004296)".
- ^ a b c Sigma-Aldrich Co., Acrylamide. Retrieved on 2022-02-15.
- ^ Cite error: The named reference
idlhwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Ullwas invoked but never defined (see the help page). - ^ "Does burnt food give you cancer?". University of Birmingham. Retrieved 2022-09-30.
- ^ Cite error: The named reference
acswas invoked but never defined (see the help page). - ^ Cite error: The named reference
crukwas invoked but never defined (see the help page).