 | ||||||||||||||||||||||||||||||||||
| Actinium | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ækˈtɪniəm/ | |||||||||||||||||||||||||||||||||
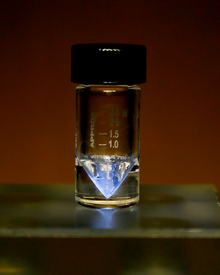
| Appearance | silvery-white, glowing with an eerie blue light;[1] sometimes with a golden cast[2] | |||||||||||||||||||||||||||||||||
| Mass number | [227] | |||||||||||||||||||||||||||||||||
| Actinium in the periodic table | ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 89 | |||||||||||||||||||||||||||||||||
| Group | f-block groups (no number) | |||||||||||||||||||||||||||||||||
| Period | period 7 | |||||||||||||||||||||||||||||||||
| Block | f-block | |||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 6d1 7s2 | |||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 9, 2 | |||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||
| Melting point | 1500 K (1227 °C, 2240 °F) (estimated)[2] | |||||||||||||||||||||||||||||||||
| Boiling point | 3500±300 K (3200±300 °C, 5800±500 °F) (extrapolated)[2] | |||||||||||||||||||||||||||||||||
| Density (near r.t.) | 10 g/cm3 | |||||||||||||||||||||||||||||||||
| Heat of fusion | 14 kJ/mol | |||||||||||||||||||||||||||||||||
| Heat of vaporization | 400 kJ/mol | |||||||||||||||||||||||||||||||||
| Molar heat capacity | 27.2 J/(mol·K) | |||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||
| Oxidation states | common: +3 | |||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.1 | |||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||
| Covalent radius | 215 pm | |||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||
| Natural occurrence | from decay | |||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc) (cF4) | |||||||||||||||||||||||||||||||||
| Lattice constant | a = 531.5 pm (at 20 °C)[3] | |||||||||||||||||||||||||||||||||
| Thermal conductivity | 12 W/(m⋅K) | |||||||||||||||||||||||||||||||||
| CAS Number | 7440-34-8 | |||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Friedrich Oskar Giesel (1902, 1903) | |||||||||||||||||||||||||||||||||
| Named by | André-Louis Debierne (1899) | |||||||||||||||||||||||||||||||||
| Isotopes of actinium | ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
Actinium is a chemical element; it has symbol Ac and atomic number 89. It was first isolated by Friedrich Oskar Giesel in 1902, who gave it the name emanium; the element got its name by being wrongly identified with a substance André-Louis Debierne found in 1899 and called actinium. The actinide series, a set of 15 elements between actinium and lawrencium in the periodic table, are named for actinium. Together with polonium, radium, and radon, actinium was one of the first non-primordial radioactive elements to be isolated.
A soft, silvery-white radioactive metal, actinium reacts rapidly with oxygen and moisture in air forming a white coating of actinium oxide that prevents further oxidation. As with most lanthanides and many actinides, actinium assumes oxidation state +3 in nearly all its chemical compounds. Actinium is found only in traces in uranium and thorium ores as the isotope 227Ac, which decays with a half-life of 21.772 years, predominantly emitting beta and sometimes alpha particles, and 228Ac, which is beta active with a half-life of 6.15 hours. One tonne of natural uranium in ore contains about 0.2 milligrams of actinium-227, and one tonne of thorium contains about 5 nanograms of actinium-228. The close similarity of physical and chemical properties of actinium and lanthanum makes separation of actinium from the ore impractical. Instead, the element is prepared, in milligram amounts, by the neutron irradiation of 226Ra in a nuclear reactor. Owing to its scarcity, high price and radioactivity, actinium has no significant industrial use. Its current applications include a neutron source and an agent for radiation therapy.
- ^ Wall, Greg (8 September 2003). "C&EN: It's Elemental: The Periodic Table - Actinium". C&EN: It's Elemental: The Periodic Table. Chemical and Engineering News. Retrieved 2 June 2011.
- ^ a b c Kirby, Harold W.; Morss, Lester R. (2006). "Actinium". The Chemistry of the Actinide and Transactinide Elements. p. 18. doi:10.1007/1-4020-3598-5_2. ISBN 978-1-4020-3555-5.
- ^ Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.

