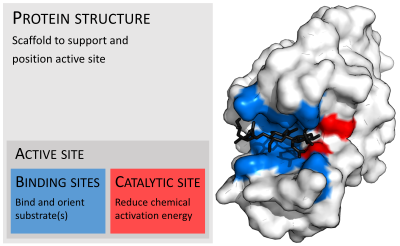
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate, the binding site, and residues that catalyse a reaction of that substrate, the catalytic site. Although the active site occupies only ~10–20% of the volume of an enzyme,[1]: 19 it is the most important part as it directly catalyzes the chemical reaction. It usually consists of three to four amino acids, while other amino acids within the protein are required to maintain the tertiary structure of the enzymes.[2]
Each active site is evolved to be optimised to bind a particular substrate and catalyse a particular reaction, resulting in high specificity. This specificity is determined by the arrangement of amino acids within the active site and the structure of the substrates. Sometimes enzymes also need to bind with some cofactors to fulfil their function. The active site is usually a groove or pocket of the enzyme which can be located in a deep tunnel within the enzyme,[3] or between the interfaces of multimeric enzymes. An active site can catalyse a reaction repeatedly as residues are not altered at the end of the reaction (they may change during the reaction, but are regenerated by the end).[4] This process is achieved by lowering the activation energy of the reaction, so more substrates have enough energy to undergo reaction.
- ^ Bugg TD (2004). Introduction to Enzyme and Coenzyme Chemistry (PDF) (2nd ed.). Blackwell Publishing Limited. ISBN 9781405114523. Archived from the original (PDF) on 22 March 2018.
- ^ Shanmugam S (2009). Enzyme Technology. I K International Publishing House. p. 48. ISBN 9789380026053.
- ^ Pravda L, Berka K, Svobodová Vařeková R, et al. (2014). "Anatomy of Enzyme Channels". BMC Bioinformatics. 15 (1): 379. doi:10.1186/s12859-014-0379-x. PMC 4245731. PMID 25403510.
- ^ Alberts B (2010). Essential Cell Biology. Garland Science. p. 91. ISBN 9780815341291.