| |
| Names | |
|---|---|
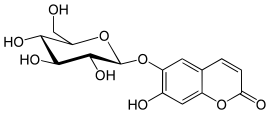
| IUPAC name
6-(β-D-Glucopyranosyloxy)-7-hydroxy-2H-1-benzopyran-2-one
| |
| Systematic IUPAC name
7-Hydroxy-6-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-2H-1-benzopyran-2-one | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.744 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H16O9 | |
| Molar mass | 340.282 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Aesculin, also called æsculin or esculin, is a coumarin glucoside that naturally occurs in the trees horse chestnut (Aesculus hippocastanum),[1] California buckeye (Aesculus californica),[2] prickly box (Bursaria spinosa), and daphnin (the dark green resin of Daphne mezereum). It is also found in dandelion coffee and olive bark.[3] It is reported to present in olive bark, not in olive leaf, therefore, identification of aesculin (coumarin derivative) in abundant in an extract indicates the extract derived from olive bark.[3]
- ^ "Plant poisons: Aesculin". University of Bristol. Retrieved July 17, 2018.
- ^ C. Michael Hogan (2008) California Buckeye: Aesculus californica, GlobalTwitcher.com, N. Stromberg ed.
- ^ a b Lo Giudice, V.; Faraone, I.; Bruno, M. R.; Ponticelli, M.; Labanca, F.; Bisaccia, D.; Massarelli, C.; Milella, L.; Todaro, L. (2021). "Olive Trees By-Products as Sources of Bioactive and Other Industrially Useful Compounds: A Systematic Review". Molecules (Basel, Switzerland). 26 (16): 5081. doi:10.3390/molecules26165081. PMC 8399450. PMID 34443669.