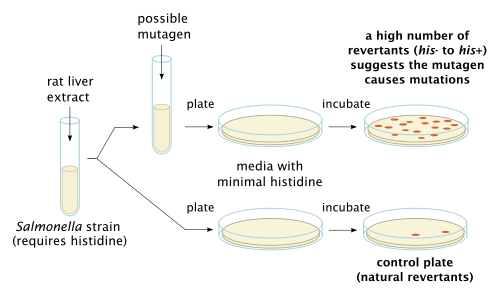
The Ames test is a widely employed method that uses bacteria to test whether a given chemical can cause mutations in the DNA of the test organism. More formally, it is a biological assay to assess the mutagenic potential of chemical compounds.[1] A positive test indicates that the chemical is mutagenic and therefore may act as a carcinogen, because cancer is often linked to mutation. The test serves as a quick and convenient assay to estimate the carcinogenic potential of a compound because standard carcinogen assays on mice and rats are time-consuming (taking two to three years to complete) and expensive. However, false-positives and false-negatives are known.[2]
The procedure was described in a series of papers in the early 1970s by Bruce Ames and his group at the University of California, Berkeley.[3][4][5][6]
- ^ Mortelmans K, Zeiger E (November 2000). "The Ames Salmonella/microsome mutagenicity assay". Mutation Research. 455 (1–2): 29–60. Bibcode:2000MRFMM.455...29M. doi:10.1016/S0027-5107(00)00064-6. PMID 11113466.
- ^ Charnley G (2002). "Ames Test". Encyclopedia of Public Health. eNotes.com. Archived from the original on 4 February 2009. Retrieved 2014-05-02.
- ^ Cite error: The named reference
ameswas invoked but never defined (see the help page). - ^ Cite error: The named reference
ames 1was invoked but never defined (see the help page). - ^ Cite error: The named reference
ames 2was invoked but never defined (see the help page). - ^ Cite error: The named reference
ames 3was invoked but never defined (see the help page).