| |

| |
| Names | |
|---|---|
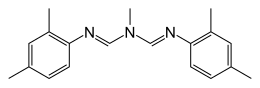
| IUPAC name
N,N'-[(Methylimino)dimethylidyne]di-2,4-xylidine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.046.691 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H23N3 | |
| Molar mass | 293.41 g/mol |
| Melting point | 86 to 87 °C (187 to 189 °F; 359 to 360 K) |
| Insoluble | |
| Vapor pressure | 2.6 x 10−6 mmHg |
| Pharmacology | |
| QP53AD01 (WHO) | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Amitraz (development code BTS27419) is a non-systemic acaricide and insecticide[1] and has also been described as a scabicide. It was first synthesized by the Boots Co. in England in 1969.[2] Amitraz has been found to have an insect repellent effect, works as an insecticide and also as a pesticide synergist.[3] Its effectiveness is traced back on alpha-adrenergic agonist activity, interaction with octopamine receptors of the central nervous system and inhibition of monoamine oxidases and prostaglandin synthesis.[4] Therefore, it leads to overexcitation and consequently paralysis and death in insects. Because amitraz is less harmful to mammals, amitraz is among many other purposes best known as insecticide against mite- or tick-infestation of dogs.[1] It is also widely used in the beekeeping industry as a control for the Varroa destructor mite, although there are recent reports of resistance (driven by overuse and off label use).[citation needed]
- ^ a b Corta, E., Bakkali, A., Berrueta, L. A., Gallo, B., & Vicente, F. (1999). Kinetics and mechanism of amitraz hydrolysis in aqueous media by HPLC and GC-MS. Talanta, 48(1), 189-199
- ^ Harrison, I. R., et al. (1973). 1,3,5-Triazapenta-1, 4-dienes: Chemical aspects of a new group of pesticides. Pestic. Sci. 4: 901
- ^ PubChem Substance. Amitraz – Substance Summary. retrieved from https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=24868774#x332
- ^ Cite error: The named reference
Bonsallwas invoked but never defined (see the help page).