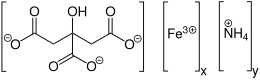
 Structure of ammonium ferric citrate
| |
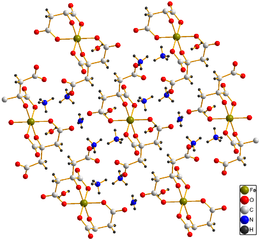 Crystal structure of [NH4]5[Fe(C6H4O7)2]·2H2O[1]
| |
| Names | |
|---|---|
| IUPAC name
2-Hydroxypropane-1,2,3-tricarboxylate, ammonium iron(3+) salt
| |
| Other names
Ferric ammonium citrate
Ammonium iron(III) citrate Ammonium ferric citrate Iron ammonium citrate FerriSeltz | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.013.351 |
| EC Number |
|
| E number | E381 (antioxidants, ...) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H8O7⋅xFe3+⋅yNH3 | |
| Appearance | yellow crystals |
| Pharmacology | |
| V08CA07 (WHO) | |
| Hazards | |
| Safety data sheet (SDS) | [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ammonium ferric citrate (also known as ferric ammonium citrate or ammoniacal ferrous citrate) has the formula [NH4]y[Fex(C6H4O7)]. The iron in this compound is trivalent. All three carboxyl groups and the central hydroxyl group of citric acid are deprotonated. A distinguishing feature of this compound is that it is very soluble in water, in contrast to ferric citrate which is not very soluble.[3]
In its crystal structure each moiety of citric acid has lost four protons. The deprotonated hydroxyl group and two of the carboxylate groups ligate to the ferric center, while the third carboxylate group coordinates with the ammonium.[1]
- ^ a b Matzapetakis, M.; Raptopoulou, C. P.; Tsohos, A.; Papaefthymiou, V.; Moon, N.; Salifoglou, A. (1998). "Synthesis, Spectroscopic and Structural Characterization of the First Mononuclear, Water Soluble Iron−Citrate Complex, (NH4)5Fe(C6H4O7)2·2H2O". J. Am. Chem. Soc. 120 (50): 13266–13267. doi:10.1021/ja9807035.
- ^ "KEGG DRUG: Ferric ammonium citrate".
- ^ PubChem. "Ammonium ferric citrate". pubchem.ncbi.nlm.nih.gov. Retrieved 4 October 2022.