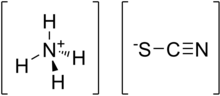
| |||
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.015.614 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3077 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| [NH4][SCN] | |||
| Molar mass | 76.122 g/mol | ||
| Appearance | Colorless hygroscopic crystalline solid | ||
| Density | 1.305 g/cm3 | ||
| Melting point | 149.5 °C (301.1 °F; 422.6 K) | ||
| Boiling point | 170 °C (338 °F; 443 K) (decomposes) | ||
| 128 g/(100 mL) (0 °C) | |||
| Solubility | soluble in liquid ammonia, alcohol, acetone | ||
| −48.1·10−6 cm3/mol | |||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Warning | |||
| H302, H312, H332, H410, H412 | |||
| P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, P391, P501 | |||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Other anions
|
Ammonium cyanate | ||
Other cations
|
|||
Related compounds
|
Ammonium cyanide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Ammonium thiocyanate is an inorganic compound with the formula [NH4]+[SCN]−. It is an ammonium salt of thiocyanic acid. It consists of ammonium cations [NH4]+ and thiocyanate anions [SCN]−.


