
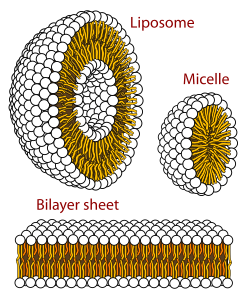
In chemistry, an amphiphile (from Greek αμφις (amphis) 'both' and φιλíα (philia) 'love, friendship'), or amphipath, is a chemical compound possessing both hydrophilic (water-loving, polar) and lipophilic (fat-loving, nonpolar) properties.[1] Such a compound is called amphiphilic or amphipathic. Amphiphilic compounds include surfactants and detergents. The phospholipid amphiphiles are the major structural component of cell membranes.
Amphiphiles are the basis for a number of areas of research in chemistry and biochemistry, notably that of lipid polymorphism.
Organic compounds containing hydrophilic groups at both ends of the molecule are called bolaamphiphilic. The micelles they form in the aggregate are prolate.
- ^ Betts, J. Gordon. "3.1 The cell membrane". Anatomy & physiology. OpenStax. ISBN 978-1-947172-04-3. Retrieved 14 May 2023.