| |

| |
| Names | |
|---|---|
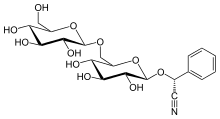
| IUPAC name
(2R)-[β-D-Glucopyranosyl-(1→6)-β-D-glucopyranosyloxy]phenylacetonitrile
| |
| Systematic IUPAC name
(2R)-Phenyl{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-({[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxan-2-yl]oxy}acetonitrile | |
| Identifiers | |
3D model (JSmol)
|
|
| 66856 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.045.372 |
| EC Number |
|
| MeSH | Amygdalin |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H27NO11 | |
| Molar mass | 457.429 |
| Melting point | 223-226 °C(lit.) |
| H2O: 0.1 g/mL hot, clear to very faintly turbid, colorless | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302 | |
| P264, P270, P301+P312, P330, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | A6005 |
| Related compounds | |
Related compounds
|
Vicianin, laetrile, prunasin, sambunigrin |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Amygdalin (from Ancient Greek: ἀμυγδαλή amygdalē 'almond') is a naturally occurring chemical compound found in many plants, most notably in the seeds (kernels, pips or stones) of apricots, bitter almonds, apples, peaches, cherries and plums, and in the roots of manioc.
Amygdalin is classified as a cyanogenic glycoside, because each amygdalin molecule includes a nitrile group, which can be released as the toxic cyanide anion by the action of a beta-glucosidase. Eating amygdalin will cause it to release cyanide in the human body, and may lead to cyanide poisoning.[1]
Since the early 1950s, both amygdalin and a chemical derivative named laetrile have been promoted as alternative cancer treatments, often under the misnomer vitamin B17 (neither amygdalin nor laetrile is a vitamin).[2] Scientific study has found them to not only be clinically ineffective in treating cancer, but also potentially toxic or lethal when taken by mouth due to cyanide poisoning.[3] The promotion of laetrile to treat cancer has been described in the medical literature as a canonical example of quackery[4][5] and as "the slickest, most sophisticated, and certainly the most remunerative cancer quack promotion in medical history".[2] It has also been described as traditional Chinese medicine.[6]
- ^ "Apricot kernels pose risk of cyanide poisoning". European Food Safety Authority. 27 April 2016.
A naturally-occurring compound called amygdalin is present in apricot kernels and converts to hydrogen cyanide after eating. Cyanide poisoning can cause nausea, fever, headaches, insomnia, thirst, lethargy, nervousness, joint and muscle various aches and pains, and falling blood pressure. In extreme cases it is fatal
- ^ a b Cite error: The named reference
CaCancerwas invoked but never defined (see the help page). - ^ Milazzo S, Horneber M (April 2015). "Laetrile treatment for cancer". The Cochrane Database of Systematic Reviews. 2015 (4): CD005476. doi:10.1002/14651858.CD005476.pub4. PMC 6513327. PMID 25918920.
- ^ Lerner IJ (February 1984). "The whys of cancer quackery". Cancer. 53 (3 Suppl): 815–819. doi:10.1002/1097-0142(19840201)53:3+<815::AID-CNCR2820531334>3.0.CO;2-U. PMID 6362828. S2CID 36332694.
- ^ Nightingale SL (1984). "Laetrile: the regulatory challenge of an unproven remedy". Public Health Reports. 99 (4): 333–338. PMC 1424606. PMID 6431478.
- ^ Shi J, Chen Q, Xu M, Xia Q, Zheng T, Teng J, et al. (June 2019). "Recent updates and future perspectives about amygdalin as a potential anticancer agent: A review". Cancer Medicine. 8 (6): 3004–3011. doi:10.1002/cam4.2197. PMC 6558459. PMID 31066207.
