| Amyloid beta peptide (beta-APP) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 A partially folded structure of amyloid beta(1 40) in an aqueous environment (pdb 2lfm)[1] | |||||||||
| Identifiers | |||||||||
| Symbol | APP | ||||||||
| Pfam | PF03494 | ||||||||
| InterPro | IPR013803 | ||||||||
| SCOP2 | 2lfm / SCOPe / SUPFAM | ||||||||
| TCDB | 1.C.50 | ||||||||
| OPM superfamily | 304 | ||||||||
| OPM protein | 2y3k | ||||||||
| Membranome | 45 | ||||||||
| |||||||||
| amyloid beta (A4) precursor protein (peptidase nexin-II, Alzheimer disease) | |||||||
|---|---|---|---|---|---|---|---|
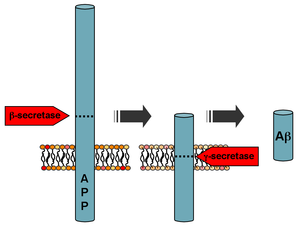 Processing of the amyloid precursor protein | |||||||
| Identifiers | |||||||
| Symbol | APP | ||||||
| Alt. symbols | AD1 | ||||||
| NCBI gene | 351 | ||||||
| HGNC | 620 | ||||||
| OMIM | 104760 | ||||||
| RefSeq | NM_000484 | ||||||
| UniProt | P05067 | ||||||
| Other data | |||||||
| Locus | Chr. 21 q21.2 | ||||||
| |||||||
Amyloid beta (Aβ or Abeta) denotes peptides of 36–43 amino acids that are the main component of the amyloid plaques found in the brains of people with Alzheimer's disease.[2] The peptides derive from the amyloid-beta precursor protein (APP), which is cleaved by beta secretase and gamma secretase to yield Aβ in a cholesterol-dependent process and substrate presentation.[3] Both neurons and oligodendrocytes produce and release Aβ in the brain, contributing to formation of amyloid plaques.[4] Aβ molecules can aggregate to form flexible soluble oligomers which may exist in several forms. It is now believed that certain misfolded oligomers (known as "seeds") can induce other Aβ molecules to also take the misfolded oligomeric form, leading to a chain reaction akin to a prion infection. The oligomers are toxic to nerve cells.[5] The other protein implicated in Alzheimer's disease, tau protein, also forms such prion-like misfolded oligomers, and there is some evidence that misfolded Aβ can induce tau to misfold.[6][7]
A study has suggested that APP and its amyloid potential is of ancient origins, dating as far back as early deuterostomes.[8]
- ^ Cite error: The named reference
Vivekanandan_2011was invoked but never defined (see the help page). - ^ Hamley IW (October 2012). "The amyloid beta peptide: a chemist's perspective. Role in Alzheimer's and fibrillization" (PDF). Chemical Reviews. 112 (10): 5147–5192. doi:10.1021/cr3000994. PMID 22813427.
- ^ Wang H, Kulas JA, Wang C, Holtzman DM, Ferris HA, Hansen SB (August 2021). "Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol". Proceedings of the National Academy of Sciences of the United States of America. 118 (33): e2102191118. Bibcode:2021PNAS..11802191W. doi:10.1073/pnas.2102191118. PMC 8379952. PMID 34385305.
- ^ Rajani RM, Ellingford R, Hellmuth M, Harris SS, Taso OS, Graykowski D, et al. (July 2024). "Selective suppression of oligodendrocyte-derived amyloid beta rescues neuronal dysfunction in Alzheimer's disease". PLOS Biology. 22 (7): e3002727. doi:10.1371/journal.pbio.3002727. PMC 11265669. PMID 39042667.
- ^ Haass C, Selkoe DJ (February 2007). "Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide". Nature Reviews. Molecular Cell Biology. 8 (2): 101–112. doi:10.1038/nrm2101. PMID 17245412. S2CID 32991755.
- ^ Nussbaum JM, Seward ME, Bloom GS (Jan–Feb 2013). "Alzheimer disease: a tale of two prions". Prion. 7 (1): 14–19. doi:10.4161/pri.22118. PMC 3609044. PMID 22965142.
- ^ Pulawski W, Ghoshdastider U, Andrisano V, Filipek S (April 2012). "Ubiquitous amyloids". Applied Biochemistry and Biotechnology. 166 (7): 1626–1643. doi:10.1007/s12010-012-9549-3. PMC 3324686. PMID 22350870.
- ^ Tharp WG, Sarkar IN (April 2013). "Origins of amyloid-β". BMC Genomics. 14 (1): 290. doi:10.1186/1471-2164-14-290. PMC 3660159. PMID 23627794.