 | |
| Clinical data | |
|---|---|
| Other names | GTx-007; S-4; Acetamidoxolutamide; Androxolutamide; Acetam-doxolutamide |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.230.653 |
| Chemical and physical data | |
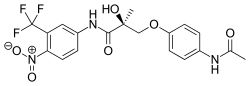
| Formula | C19H18F3N3O6 |
| Molar mass | 441.363 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Andarine (developmental code names GTx-007, S-4) is a selective androgen receptor modulator (SARM) which was developed by GTX, Inc for the treatment of conditions such as muscle wasting, osteoporosis, and benign prostatic hypertrophy (BPH),[1] using the nonsteroidal antiandrogen bicalutamide as a lead compound.[2] Development of andarine for all indications has been discontinued, in favor of the structurally related and improved compound enobosarm (ostarine; GTx-024; S-22).[3]
Andarine is an orally active partial agonist of the androgen receptor (AR). In intact male rats, 0.5 mg andarine daily was shown to reduce prostate weight to 79.4%, and non-significantly increased levator ani muscle weight. In castrated male rats, this dose restored only 32.5% prostate weight, but 101% levator ani muscle weight [4] This suggests that andarine is able to competitively block binding of dihydrotestosterone to its receptor targets in the prostate gland, but its partial agonist actions at the androgen receptor prevent the side effects associated with the antiandrogens traditionally used for treatment of BPH.[5]
Andarine was first described in the literature by 2002.[6][7][8] It completed phase 1 clinical trials for cachexia in 2003.[9][10] Three phase 1 trials (1a, 1b, 1c) were completed with the drug involving 86 healthy male and female volunteers.[10] Phase 2 trials were planned for 2004.[10] However, development of andarine was discontinued, reportedly due to findings of visual disturbances in clinical studies.[3][11] Andarine is thought to have been the first SARM to enter human clinical trials.[12]
- ^ Yin D, Gao W, Kearbey JD, Xu H, Chung K, He Y, et al. (March 2003). "Pharmacodynamics of selective androgen receptor modulators". The Journal of Pharmacology and Experimental Therapeutics. 304 (3). American Society for Pharmacology & Experimental Therapeutics (ASPET): 1334–1340. doi:10.1124/jpet.102.040840. PMID 12604714. S2CID 14724811.
- ^ Chen J, Kim J, Dalton JT (June 2005). "Discovery and therapeutic promise of selective androgen receptor modulators". Molecular Interventions. 5 (3): 173–188. doi:10.1124/mi.5.3.7. PMC 2072877. PMID 15994457.
- ^ a b "Andarine". AdisInsight. Springer Nature Switzerland AG.
- ^ GGao W, Kearbey JD, Nair VA, Chung K, Parlow AF, Miller DD, Dalton JT (December 2004). "Comparison of the pharmacological effects of a novel selective androgen receptor modulator, the 5alpha-reductase inhibitor finasteride, and the antiandrogen hydroxyflutamide in intact rats: new approach for benign prostate hyperplasia". Endocrinology. 145 (12): 5420–5428. doi:10.1210/en.2004-0627. PMC 2098692. PMID 15308613.
- ^ Gao W, Kim J, Dalton JT (August 2006). "Pharmacokinetics and pharmacodynamics of nonsteroidal androgen receptor ligands". Pharmaceutical Research. 23 (8): 1641–1658. doi:10.1007/s11095-006-9024-3. PMC 2072875. PMID 16841196.
- ^ He Y, Yin D, Perera M, Kirkovsky L, Stourman N, Li W, et al. (August 2002). "Novel nonsteroidal ligands with high binding affinity and potent functional activity for the androgen receptor". European Journal of Medicinal Chemistry. 37 (8): 619–634. doi:10.1016/s0223-5234(02)01335-1. PMID 12161060.
- ^ Yin D, Gao W, Kearbey JD, Xu H, Chung K, He Y, et al. (March 2003). "Pharmacodynamics of selective androgen receptor modulators". The Journal of Pharmacology and Experimental Therapeutics. 304 (3): 1334–1340. doi:10.1124/jpet.102.040840. PMID 12604714. S2CID 14724811.
- ^ Perera MA (2003). The pharmacology, pharmacokinetics and metabolism of a novel nonsteroidal selective androgen receptor modulator (Ph.D. thesis). OCLC 56700020. ProQuest 305301414.[page needed]
- ^ Mohler ML, Bohl CE, Jones A, Coss CC, Narayanan R, He Y, et al. (June 2009). "Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit". Journal of Medicinal Chemistry. 52 (12): 3597–3617. doi:10.1021/jm900280m. PMID 19432422.
The peripheral and selective anabolic preclinical pharmacodynamic profile of 8 seemed highly promising and 3602 Journal of Medicinal Chemistry, 2009, Vol. 52, No. 12 Award Address stimulated us to pursue landmark clinical trials of the SARMs, andarine 8 and Ostarine.75 Although phase I studies with 8 were successful with no deficiencies noted (March 17, 2004, press release), Ostarine was selected for advanced clinical development based on corporate strategy. Readers are cautioned to note that the name Ostarine is often mistakenly linked to the chemical structure of 8, which is also known as andarine. The chemical structure of Ostarine has not been publicly disclosed. The authors are unable to provide additional information.
- ^ a b c "Form S-1: GTx, Inc". U.S. Securities and Exchange Commission. 22 December 2003.
Clinical Trials. We have completed three Phase I clinical trials of Andarine in a total of 86 healthy male and female volunteers. We tested Andarine for safety and tolerance in single and multiple doses. Results from our Phase I trials support once-a-day oral dosing, and no serious adverse events were observed at any single or multiple dose tested. We observed early indications in the multiple-dose Phase I clinical trial in men that Andarine promoted growth activity, as measured by levels of a growth factor in the blood known as IGF-1, without affecting the sebaceous glands. We believe that these observations support the potential ability of Andarine to selectively modulate androgen receptors in a tissue-specific manner.
- ^ Starcevic B, Ahrens BD, Butch AW (May 2013). "Detection of the selective androgen receptor modulator S-4 (Andarine) in a doping control sample". Drug Testing and Analysis. 5 (5): 377–379. doi:10.1002/dta.1466. PMID 23427117.
S-4 [Andarine, S3-(4-acetylamino-phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamide] a member of the aryl propionamide class of SARMs was evaluated in a phase I clinical trial, but the study had to be stopped due to adverse side-effects involving visual disturbances.[3]
- ^ "Form 8-K". U.S. Securities and Exchange Commission. 3 March 2004 – via Oncternal Therapeutics, Inc.
We believe Andarine represents the first SARM to enter human clinical trials.