| |

| |
| Names | |
|---|---|
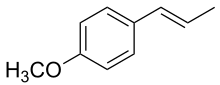
| Preferred IUPAC name
1-Methoxy-4-[(E)-prop-1-enyl]benzene[1] | |
| Other names
(E)-1-Methoxy-4-(prop-1-en-1-yl)benzene
(E)-1-Methoxy-4-(1-propenyl)benzene para-Methoxyphenylpropene p-Propenylanisole Isoestragole trans-1-Methoxy-4-(prop-1-enyl)benzene | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.914 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H12O | |
| Molar mass | 148.205 g/mol |
| Density | 0.998 g/cm3 |
| Melting point | 20 to 21 °C (68 to 70 °F; 293 to 294 K) |
| Boiling point | 234 °C (453 °F; 507 K) 81 °C (178 °F; 354 K) at 2 mmHg |
| −9.60×10−5 cm3/mol | |
| Hazards | |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related compounds
|
anisole estragole |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Anethole (also known as anise camphor)[2] is an organic compound that is widely used as a flavoring substance. It is a derivative of the aromatic compound allylbenzene and occurs widely in the essential oils of plants. It is in the class of phenylpropanoid organic compounds. It contributes a large component of the odor and flavor of anise and fennel (both in the botanical family Apiaceae), anise myrtle (Myrtaceae), liquorice (Fabaceae), magnolia blossoms, and star anise (Schisandraceae). Closely related to anethole is its isomer estragole, which is abundant in tarragon (Asteraceae) and basil (Lamiaceae), and has a flavor reminiscent of anise. It is a colorless, fragrant, mildly volatile liquid.[clarification needed][3] Anethole is only slightly soluble in water but exhibits high solubility in ethanol. This trait causes certain anise-flavored liqueurs to become opaque when diluted with water; this is called the ouzo effect.
- ^ "Anethole".
- ^ "Anise camphor definition and meaning | Collins English Dictionary".
- ^ Fahlbusch, Karl-Georg; Hammerschmidt, Franz-Josef; Panten, Johannes; Pickenhagen, Wilhelm; Schatkowski, Dietmar; Bauer, Kurt; Garbe, Dorothea; Surburg, Horst. "Flavors and Fragrances". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_141. ISBN 978-3527306732.