 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Eliquis, others |
| Other names | BMS-562247-01 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613032 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~50% |
| Protein binding | ~87% |
| Metabolism | CYP3A4, CYP3A5, CYP1A2 and others |
| Elimination half-life | 9–14 h |
| Excretion | Bile duct (75%), kidney (25%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.167.332 |
| Chemical and physical data | |
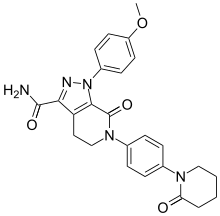
| Formula | C25H25N5O4 |
| Molar mass | 459.506 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Apixaban, sold under the brand name Eliquis, is an anticoagulant medication used to treat and prevent blood clots and to prevent stroke in people with nonvalvular atrial fibrillation through directly inhibiting factor Xa.[6][7][8] It is used as an alternative to warfarin to prevent blood clots following hip or knee replacement and in those with a history of prior clots.[6][8] and does not require monitoring by blood tests[6] or dietary restrictions.[9] It is taken by mouth.[6]
Common side effects include bleeding and nausea.[6][7] Other side effects may include bleeding around the spine and allergic reactions.[6] Use is not recommended during pregnancy or breastfeeding.[1][7] Use appears to be relatively safe in those with mild kidney problems.[7] Compared to warfarin it has fewer interactions with other medications.[10] It is a direct factor Xa inhibitor.[6]
In 2007, Pfizer and Bristol-Myers Squibb began development of apixaban as an anticoagulant.[11] Apixaban was approved for medical use in the European Union in May 2011, and in the United States in December 2012.[5][6][12] It is on the World Health Organization's List of Essential Medicines.[13] In 2022, it was the 27th most commonly prescribed medication in the United States, with more than 19 million prescriptions.[14][15] It is available as a generic medication, although not in the United States.[8][16]
- ^ a b "Apixaban (Eliquis) Use During Pregnancy". Drugs.com. 21 June 2019. Retrieved 13 August 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Eliquis 5 mg film-coated tablets - Summary of Product Characteristics (SmPC)". (emc). 3 May 2022. Retrieved 8 October 2022.
- ^ Cite error: The named reference
Eliquis FDA labelwas invoked but never defined (see the help page). - ^ a b "Eliquis EPAR". European Medicines Agency. 17 September 2018. Retrieved 22 April 2020. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b c d e f g h "Apixaban Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 27 March 2019.
- ^ a b c d British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 124–125. ISBN 9780857113382.
- ^ a b c "FDA approves first generics of Eliquis". U.S. Food and Drug Administration (FDA). 23 December 2019. Archived from the original on 23 December 2019. Retrieved 23 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "How a Drug Is Born". Skeptical Inquirer. Amherst, New York: Center for Inquiry. September–October 2020.
{{cite magazine}}: Unknown parameter|authors=ignored (help) - ^ Kiser K (2017). Oral Anticoagulation Therapy: Cases and Clinical Correlation. Springer. p. 11. ISBN 9783319546438.
- ^ "Bristol-Myers Squibb and Pfizer Announce Worldwide Collaboration to Develop and Commercialize Anticoagulant and Metabolic Compounds". Pfizer (Press release). Archived from the original on 10 September 2015. Retrieved 25 December 2021.
- ^ "Drug Approval Package: Eliquis (apixaban) NDA #202155". U.S. Food and Drug Administration (FDA). 13 February 2013. Retrieved 23 December 2019.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Apixaban Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ "With Court Win, BMS and Pfizer Stave Off Generic Challengers to Eliquis – For Now". BioSpace. 6 August 2020. Retrieved 29 November 2021.