 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Emend, Cinvanti, Aponvie, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604003 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | NK1 receptor antagonist, antiemetic |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60–65% |
| Protein binding | >95% |
| Metabolism | Liver (mostly CYP3A4- mediated; some contributions by CYP2C19 & CYP1A2) |
| Elimination half-life | 9–13 hours |
| Excretion | Kidney (57%), feces (45%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.202.762 |
| Chemical and physical data | |
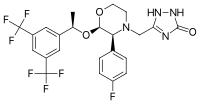
| Formula | C23H21F7N4O3 |
| Molar mass | 534.435 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Aprepitant, sold under the brand name Emend among others, is a medication used to prevent chemotherapy-induced nausea and vomiting and to prevent postoperative nausea and vomiting.[5] It may be used together with ondansetron and dexamethasone.[5] It is taken by mouth[5] or administered by intravenous injection.[3]
Common side effects include tiredness, loss of appetite, diarrhea, abdominal pain, hiccups, itchiness, pneumonia, and blood pressure changes.[5] Other severe side effects may include anaphylaxis.[5] While use in pregnancy does not appear to be harmful, such use has not been well studied.[6] Aprepitant belongs to the class of neurokinin-1 receptor antagonists.[5] It works by blocking substance P from attaching to the NK1 receptors.[4]
Aprepitant was approved for medical use in the European Union and the United States in 2003.[5][4] It is made by Merck & Co.[5] It is on the World Health Organization's List of Essential Medicines.[7][8]
- ^ "Emend- aprepitant capsule Emend- aprepitant kit Emend- aprepitant powder, for suspension". DailyMed. 6 May 2022. Archived from the original on 9 March 2022. Retrieved 27 September 2022.
- ^ "Cinvanti- aprepitant injection, emulsion". DailyMed. 24 March 2022. Archived from the original on 31 August 2022. Retrieved 27 September 2022.
- ^ a b "Aponvie (aprepitant) injectable emulsion, for intravenous use Initial U.S. Approval: 2003" (PDF). Archived (PDF) from the original on 28 September 2022. Retrieved 28 September 2022.
- ^ a b c "Emend EPAR". European Medicines Agency. 17 September 2018. Archived from the original on 12 November 2020. Retrieved 13 October 2019.
- ^ a b c d e f g h "Aprepitant/Fosaprepitant Dimeglumine Monograph for Professionals". Drugs.com. Archived from the original on 13 August 2020. Retrieved 13 October 2019.
- ^ "Aprepitant Use During Pregnancy". Drugs.com. Archived from the original on 28 October 2020. Retrieved 13 October 2019.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.