| |
| Names | |
|---|---|
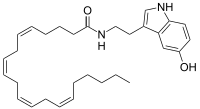
| Preferred IUPAC name
(5Z,8Z,11Z,14Z)-N-[2-(5-Hydroxy-1H-indol-3-yl)ethyl]icosa-5,8,11,14-tetraenamide | |
| Other names
N-arachidonoyl-serotonin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H42N2O2 | |
| Molar mass | 462.678 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Arachidonoyl serotonin (N-arachidonoyl-serotonin, AA-5-HT) is an endogenous lipid signaling molecule. It was first described in 1998 as being an inhibitor of fatty acid amide hydrolase (FAAH).[1] In 2007, it was shown to have analgesic properties and to act as an antagonist of the TRPV1 receptor.[2] In 2011, it was shown to be present in the ileum and jejunum of the gastrointestinal tract and modulate glucagon-like peptide-1 (GLP-1) secretion.[3] In addition to this, in 2016, AA-5-HT was also found to affect the signaling mechanisms responsible for anxiety, by inhibiting dopamine release from the Basolateral amygdala following fear behavior.[4] In 2017, AA-5-HT was tested in its effects on the sleep wake cycle, where it was found to affect the sleep homeostasis when used in conjunction with molecules and chemicals that affect wake-related neurotransmitters.[5]
- ^ Bisogno, T.; Melck, D.; De Petrocellis, L.; Bobrov, M. U.; Gretskaya, N. M.; Bezuglov, V. V.; Sitachitta, N.; Gerwick, W. H.; Di Marzo, V. (1998). "Arachidonoylserotonin and other novel inhibitors of fatty acid amide hydrolase". Biochemical and Biophysical Research Communications. 248 (3): 515–522. doi:10.1006/bbrc.1998.8874. PMID 9703957.
- ^ Maione, S.; De Petrocellis, L.; De Novellis, V.; Moriello, A. S.; Petrosino, S.; Palazzo, E.; Rossi, F. S.; Woodward, D. F.; Di Marzo, V. (2007). "Analgesic actions of N-arachidonoyl-serotonin, a fatty acid amide hydrolase inhibitor with antagonistic activity at vanilloid TRPV1 receptors". British Journal of Pharmacology. 150 (6): 766–781. doi:10.1038/sj.bjp.0707145. PMC 2013858. PMID 17279090.
- ^ Verhoeckx, K. C. M.; Voortman, T.; Balvers, M. G. J.; Hendriks, H. F. J.; m.Wortelboer, H.; Witkamp, R. F. (2011). "Presence, formation and putative biological activities of N-acyl serotonins, a novel class of fatty-acid derived mediators, in the intestinal tract". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1811 (10): 578–586. doi:10.1016/j.bbalip.2011.07.008. PMID 21798367.
- ^ Freels, Timothy G.; Lester, Deranda B.; Cook, Melloni N. (2019-04-19). "Arachidonoyl serotonin (AA-5-HT) modulates general fear-like behavior and inhibits mesolimbic dopamine release". Behavioural Brain Research. 362: 140–151. doi:10.1016/j.bbr.2019.01.010. ISSN 0166-4328. PMID 30639609. S2CID 58578695.
- ^ Murillo-Rodríguez, Eric; Di Marzo, Vincenzo; Machado, Sergio; Rocha, Nuno B.; Veras, André B.; Neto, Geraldo A. M.; Budde, Henning; Arias-Carrión, Oscar; Arankowsky-Sandoval, Gloria (2017). "Role of N-Arachidonoyl-Serotonin (AA-5-HT) in Sleep-Wake Cycle Architecture, Sleep Homeostasis, and Neurotransmitters Regulation". Frontiers in Molecular Neuroscience. 10: 152. doi:10.3389/fnmol.2017.00152. ISSN 1662-5099. PMC 5447686. PMID 28611585.