 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌætəˈzænəvɪər/ AT-ə-ZAN-ə-veer[1] |
| Trade names | Reyataz, Evotaz, others[2] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603019 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60-68% |
| Protein binding | 86% |
| Metabolism | Liver (CYP3A4-mediated) |
| Elimination half-life | 6.5 hours |
| Excretion | Fecal and kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.243.594 |
| Chemical and physical data | |
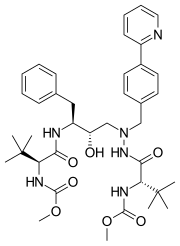
| Formula | C38H52N6O7 |
| Molar mass | 704.869 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Atazanavir, sold under the brand name Reyataz among others, is an antiretroviral medication used to treat HIV/AIDS.[2] It is generally recommended for use with other antiretrovirals.[2] It may be used for prevention after a needlestick injury or other potential exposure (postexposure prophylaxis (PEP)).[2] It is taken by mouth.[2]
Common side effects include headache, nausea, yellowish skin, abdominal pain, trouble sleeping, and fever.[2] Severe side effects include rashes such as erythema multiforme and high blood sugar.[2] Atazanavir appears to be safe to use during pregnancy.[2] It is of the protease inhibitor (PI) class and works by blocking HIV protease.[2]
Atazanavir was approved for medical use in the United States in 2003.[2] It is on the World Health Organization's List of Essential Medicines.[9] It is available as a generic medication.[10]
- ^ "Atazanavir". MedlinePlus. National Institutes of Health. 15 October 2012. Archived from the original on 2 August 2013. Retrieved 3 August 2013.
- ^ a b c d e f g h i j "Atazanavir Sulfate". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 28 November 2016.
- ^ "Atazanavir (Reyataz) Use During Pregnancy". Drugs.com. 27 February 2020. Archived from the original on 1 October 2022. Retrieved 15 September 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Archived from the original on 6 July 2023. Retrieved 30 March 2024.
- ^ "Product monograph brand safety updates". Health Canada. 7 July 2016. Archived from the original on 29 March 2024. Retrieved 3 April 2024.
- ^ "Reyataz- atazanavir capsule, gelatin coated; Reyataz- atazanavir powder". DailyMed. 7 November 2023. Retrieved 17 October 2024.
- ^ "Reyataz EPAR". European Medicines Agency (EMA). 2 March 2004. Archived from the original on 3 December 2023. Retrieved 25 May 2024.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "Teva Announces Exclusive Launch of a Generic Version of Reyataz in the United States" (Press release). Teva. 27 December 2017. Archived from the original on 4 October 2022. Retrieved 29 September 2021 – via Business Wire.