 | |
| Clinical data | |
|---|---|
| Trade names | Vidaza, Azadine, Onureg |
| Other names | 5-Azacytidine, Azacytidine, Ladakamycin, 4-Amino-1-β-D-ribofuranosyl-s-triazin-2(1H)-one, U-18496, CC-486 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607068 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Subcutaneous, intravenous, by mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 4 hr.[8] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.711 |
| Chemical and physical data | |
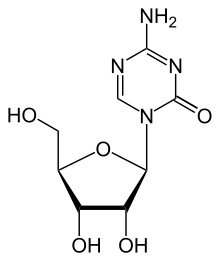
| Formula | C8H12N4O5 |
| Molar mass | 244.207 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Azacitidine, sold under the brand name Vidaza among others, is a medication used for the treatment of myelodysplastic syndrome, myeloid leukemia,[5][6] and juvenile myelomonocytic leukemia.[4][9] It is a chemical analog of cytidine, a nucleoside in DNA and RNA.[medical citation needed] Azacitidine and its deoxy derivative, decitabine (also known as 5-aza-2′-deoxycytidine) were first synthesized in Czechoslovakia as potential chemotherapeutic agents for cancer.[10]
The most common adverse reactions in children with juvenile myelomonocytic leukemia include pyrexia, rash, upper respiratory tract infection, and anemia.[9]
- ^ "Azacitidine (Vidaza) Use During Pregnancy". Drugs.com. 5 May 2020. Retrieved 12 August 2020.
- ^ "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 30 March 2024.
- ^ "Health product highlights 2021: Annexes of products approved in 2021". Health Canada. 3 August 2022. Retrieved 25 March 2024.
- ^ a b Cite error: The named reference
Vidaza FDA labelwas invoked but never defined (see the help page). - ^ a b "Onureg- azacitidine tablet, film coated". DailyMed. 20 May 2021. Retrieved 24 May 2022.
- ^ a b "Onureg EPAR". European Medicines Agency. 20 April 2021. Retrieved 6 September 2021.
- ^ "Onureg Product information". Union Register of medicinal products. Retrieved 3 March 2023.
- ^ Vallerand AH, Deglin JH (2009). Davis's drug guide for nurses. Philadelphia: F.A. Davis Company. pp. 204–206. ISBN 978-0-8036-1912-8.
- ^ a b "FDA approves azacitidine". U.S. Food and Drug Administration. 20 May 2022. Retrieved 24 May 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Cihák A (1974). "Biological effects of 5-azacytidine in eukaryotes". Oncology. 30 (5): 405–22. doi:10.1159/000224981. PMID 4142650.