| |||
| Clinical data | |||
|---|---|---|---|
| Trade names | Olumiant, others | ||
| Other names | INCB28050, LY3009104 | ||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a618033 | ||
| License data |
| ||
| Pregnancy category | |||
| Routes of administration | By mouth | ||
| ATC code | |||
| Legal status | |||
| Legal status | |||
| Pharmacokinetic data | |||
| Bioavailability | 79% | ||
| Protein binding | 50% | ||
| Metabolism | CYP3A4 (<10%) | ||
| Elimination half-life | 12.5 hours | ||
| Excretion | 75% urine, 20% faeces | ||
| Identifiers | |||
| |||
| CAS Number | |||
| PubChem CID | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| PDB ligand | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.219.080 | ||
| Chemical and physical data | |||
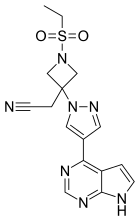
| Formula | C16H17N7O2S | ||
| Molar mass | 371.42 g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
Baricitinib, sold under the brand name Olumiant among others, is an immunomodulatory medication used for the treatment of rheumatoid arthritis, alopecia areata, and COVID-19.[7][8][9][10] It acts as an inhibitor of janus kinase (JAK), blocking the subtypes JAK1 and JAK2.[11]
Baricitinib is approved for medical use in the European Union[8] and in the United States.[9][12][10]
- ^ a b "Olumiant Product Information" (PDF). Therapeutic Goods Administration (TGA). Archived from the original on 20 September 2021. Retrieved 12 June 2021.
- ^ "Baricitinib (Olumiant) Use During Pregnancy". Drugs.com. 8 November 2019. Archived from the original on 26 June 2020. Retrieved 16 March 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "AusPAR: Baricitinib". Therapeutic Goods Administration (TGA). 20 May 2021. Archived from the original on 20 May 2021. Retrieved 11 June 2021.
- ^ "Summary Basis of Decision (SBD) for Olumiant". Health Canada. 23 October 2014. Archived from the original on 31 May 2022. Retrieved 29 May 2022.
- ^ "Regulatory Decision Summary for Olumiant". Drug and Health Products Portal. 26 January 2024. Retrieved 2 April 2024.
- ^ a b Cite error: The named reference
Olumiant FDA labelwas invoked but never defined (see the help page). - ^ a b c "Olumiant EPAR". European Medicines Agency (EMA). 3 December 2019. Archived from the original on 25 August 2021. Retrieved 17 March 2020. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b "Drug Trials Snapshots: Olumiant". U.S. Food and Drug Administration (FDA). 31 May 2018. Archived from the original on 13 December 2019. Retrieved 16 March 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "FDA Approves First Systemic Treatment for Alopecia Areata". U.S. Food and Drug Administration (FDA) (Press release). 13 June 2022. Archived from the original on 14 June 2022. Retrieved 13 June 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Summary of opinion for Olumiant" (PDF). European Medicines Agency (EMA). 15 December 2016. Archived (PDF) from the original on 15 March 2018. Retrieved 18 December 2016.
- ^ "Drug Approval Package: Olumiant (baricitinib)". U.S. Food and Drug Administration (FDA). 5 July 2018. Archived from the original on 28 April 2020. Retrieved 16 March 2020.