| Beckmann rearrangement | |
|---|---|
| Named after | Ernst Otto Beckmann |
| Reaction type | Rearrangement reaction |
| Identifiers | |
| Organic Chemistry Portal | beckmann-rearrangement |
| RSC ontology ID | RXNO:0000026 |
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement of an oxime functional group to substituted amides.[1][2] The rearrangement has also been successfully performed on haloimines and nitrones. Cyclic oximes and haloimines yield lactams.
The Beckmann rearrangement is often catalyzed by acid; however, other reagents have been known to promote the rearrangement. These include tosyl chloride, thionyl chloride, phosphorus pentachloride, phosphorus pentoxide, triethylamine, sodium hydroxide, trimethylsilyl iodide among others.[3] The Beckmann fragmentation is another reaction that often competes with the rearrangement, though careful selection of promoting reagent and solvent conditions can favor the formation of one over the other, sometimes giving almost exclusively one product. The rearrangement occurs stereospecifically for ketoximes and N-chloro/N-fluoro imines, with the migrating group being anti-periplanar to the leaving group on the nitrogen. Certain conditions have been known to racemize the oxime geometry, leading to the formation of both regioisomers. The rearrangement of aldoximes occurs with stereospecificity in the gas phase and without stereospecificity in the solution phase. A few methodologies allow for the rearrangement of aldoximes to primary amides, but fragmentation commonly competes in these systems. Nitrone rearrangement also occurs without stereospecificity; the regioisomer formed has the amide nitrogen substituted with the group possessing the greatest migratory aptitude.
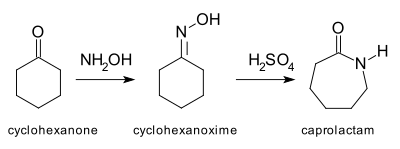
The archetypal Beckmann rearrangement[4] is the conversion of cyclohexanone to caprolactam via the oxime. Caprolactam is the feedstock in the production of Nylon 6.[5]
The Beckmann solution consists of acetic acid, hydrochloric acid and acetic anhydride, and was widely used to catalyze the rearrangement. Other acids, such as sulfuric acid, polyphosphoric acid, and hydrogen fluoride have all been used. Sulfuric acid is the most commonly used acid for commercial lactam production due to its formation of an ammonium sulfate by-product when neutralized with ammonia. Ammonium sulfate is a common agricultural fertilizer providing nitrogen and sulfur.
- ^ Ernst Otto Beckmann (1886). "Zur Kenntniss der Isonitrosoverbindungen" [On [our] knowledge of isonitroso compounds]. Berichte der Deutschen Chemischen Gesellschaft. 19: 988–993. doi:10.1002/cber.188601901222.
- ^ Donaruma, L. G.; Heldt, W. Z. (1960). "The Beckmann rearrangement. (Review)". Org. React. 11: 1–156.
- ^ Gawley, R. E. (1988). "The Beckmann reactions: rearrangement, elimination-additions, fragmentations, and rearrangement-cyclizations. (Review)". Org. React. 35: 14–24.
- ^ Eck, J. C.; Marvel, C. S. (1939). "Ε-Benzoylaminocaproic Acid". Organic Syntheses. 19: 20. doi:10.15227/orgsyn.019.0020. Archived from the original on 2012-09-28. Retrieved 2005-08-18. Eck, J. C.; Marvel, C. S. (1943). "Ε-Benzoylaminocaproic Acid". Organic Syntheses. 2: 76. Archived from the original on 2012-09-28. Retrieved 2005-08-18.
- ^ Josef Ritz; Hugo Fuchs; Heinz Kieczka; William C. Moran. "Caprolactam". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_031.pub2. ISBN 978-3527306732.