 | |
| Clinical data | |
|---|---|
| Trade names | Sirturo |
| Other names | Bedaquiline fumarate,[1] TMC207,[2] R207910, AIDS222089 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613022 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | >99.9%[6] |
| Metabolism | Liver, by CYP3A4[7] |
| Elimination half-life | 5.5 months[7] |
| Excretion | fecal[7] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
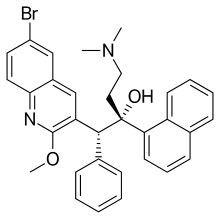
| Formula | C32H31BrN2O2 |
| Molar mass | 555.516 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Bedaquiline, sold under the brand name Sirturo, is a medication used for the treatment of active tuberculosis.[1] Specifically, it is used to treat multi-drug-resistant tuberculosis along with other medications for tuberculosis.[1][8][9] It is taken by mouth.[3]
Common side effects include nausea, joint pains, headaches, and chest pain.[1] Serious side effects include QT prolongation, liver dysfunction, and an increased risk of death.[1] While harm during pregnancy has not been found, it has not been well studied in this population.[10] It is in the diarylquinoline antimycobacterial class of medications.[1] It works by blocking the ability of M. tuberculosis to make adenosine 5'-triphosphate (ATP).[1]
Bedaquiline was approved for medical use in the United States in 2012.[1] It is on the World Health Organization's List of Essential Medicines.[11]
- ^ a b c d e f g h "Bedaquiline Fumarate". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- ^ Diacon AH, Pym A, Grobusch M, Patientia R, Rustomjee R, Page-Shipp L, et al. (June 2009). "The diarylquinoline TMC207 for multidrug-resistant tuberculosis". The New England Journal of Medicine. 360 (23): 2397–405. doi:10.1056/NEJMoa0808427. PMID 19494215.
- ^ a b "Sirturo- bedaquiline fumarate tablet". DailyMed. 17 October 2023. Archived from the original on 19 April 2024. Retrieved 20 April 2024.
- ^ "Sirturo EPAR". European Medicines Agency. 26 August 2005. Archived from the original on 18 March 2024. Retrieved 18 March 2024.
- ^ "Sirturo Product information". Union Register of medicinal products. 7 March 2014. Archived from the original on 18 March 2024. Retrieved 18 March 2024.
- ^ "Sirturo: Clinical Pharmacology". Archived from the original on 28 February 2015. Retrieved 28 April 2014.
- ^ a b c "Bedaquiline". Archived from the original on 20 May 2013. Retrieved 28 April 2014.
- ^ "WHO Rapid Communication: Key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB)". World Health Organization (WHO). Archived from the original on 20 August 2018. Retrieved 24 April 2019.
- ^ Ahmad N, Ahuja SD, Akkerman OW, Alffenaar JC, Anderson LF, Baghaei P, et al. (Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment–2017) (September 2018). "Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis". Lancet. 392 (10150): 821–834. doi:10.1016/S0140-6736(18)31644-1. PMC 6463280. PMID 30215381.
- ^ "Bedaquiline (Sirturo) Use During Pregnancy". www.drugs.com. Archived from the original on 20 December 2016. Retrieved 10 December 2016.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.