| |
| Names | |
|---|---|
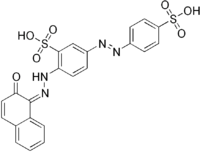
| IUPAC name
2-[(2Z)-2-(2-oxonaphthalen-1-ylidene)hydrazinyl]-5-(4-sulfophenyl)diazenyl-benzenesulfonic acid
| |
| Other names
Croceine scarlet
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.021.895 |
| EC Number |
|
| MeSH | Biebrich+scarlet |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C22H16N4O7S2 | |
| Molar mass | 512.517 |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H312, H315, H319, H332, H335 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Biebrich scarlet (C.I. 26905) is a molecule used in Lillie's trichrome.[1]
The dye was created in 1878 by the German chemist Rudolf Nietzki.[2]
Biebrich scarlet dyes are used to color hydrophobic materials like fats and oils.[3] The dye is an illegal dye for food additives because of its carcinogenic properties. Biebrich scarlet can have harmful effects on living and non-living organisms in natural water, therefore the pollutant must be removed. Removal of the pollutant involves absorption, membrane filtration, precipitation, ozonation, fungal detachment, and electrochemical separation.[3] Hydrogel absorbents have active sites to which the dye is held using electrostatic interactions. Photocatalysis allows for almost total degradation of Biebrich scarlet azo dye bonds in less than 10 hours.[4] Degradation of Biebrich scarlet is also observed using lignin peroxidase enzyme from wood rotting fungus in the presence of mediators like 2-chloro-1,4-dimethoxybenzene.[5]
- ^ Lillie, R. D. (1940). "Further Experiments with the Masson Trichrome Modification of Mallory's Connective Tissue Stain". Stain Technology. 15 (1): 17–22. doi:10.3109/10520294009110327.
- ^ Schwarz, Holm-Dietmar (1999). "Nietzki, Rudolf Hugo". Neue Deutsche Biographie (in German). Vol. 19. Retrieved 2015-10-12.
- ^ a b Priya; Sharma, Amit Kumar; Kaith, Balbir Singh; et al. (2020). "Chemically modified chitosan‑sodium alginate as chemo-sensor adsorbent for the detection of picric acid and removal of biebrich scarlet". International Journal of Biological Macromolecules. 147: 582–594. doi:10.1016/j.ijbiomac.2020.01.090. PMID 31945433. S2CID 210699599.
- ^ Chebli, D.; Fourcade, F.; Brosillon, S.; et al. (12 January 2010). "Supported photocatalysis as a pre-treatment prior to biological degradation for the removal of some dyes from aqueous solutions; Acid Red 183, Biebrich Scarlet, Methyl Red Sodium Salt, Orange II". Journal of Chemical Technology & Biotechnology. doi:10.1002/jctb.2342.
- ^ Cooksey, CJ. (2020-04-02). "Quirks of dye nomenclature. 13. Biebrich scarlet". Biotechnic & Histochemistry. 95 (3): 194–197. doi:10.1080/10520295.2019.1662945. ISSN 1052-0295. PMID 31592687. S2CID 203925217.