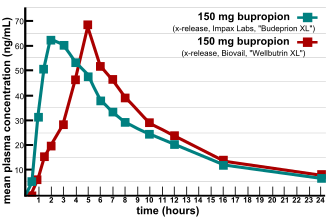
Bioequivalence is a term in pharmacokinetics used to assess the expected in vivo biological equivalence of two proprietary preparations of a drug. If two products are said to be bioequivalent it means that they would be expected to be, for all intents and purposes, the same.
One article defined bioequivalence by stating that, "two pharmaceutical products are bioequivalent if they are pharmaceutically equivalent and their bioavailabilities (rate and extent of availability) after administration in the same molar dose are similar to such a degree that their effects, with respect to both efficacy and safety, can be expected to be essentially the same. Pharmaceutical equivalence implies the same amount of the same active substance(s), in the same dosage form, for the same route of administration and meeting the same or comparable standards."[1]
For The World Health Organization (WHO) "two pharmaceutical products are bioequivalent if they are pharmaceutically equivalent or pharmaceutical alternatives, and their bioavailabilities, in terms of rate (Cmax and tmax) and extent of absorption (area under the curve), after administration of the same molar dose under the same conditions, are similar to such a degree that their effects can be expected to be essentially the same".[2]
The United States Food and Drug Administration (FDA) has defined bioequivalence as, "the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study."[3]
- ^ Birkett DJ (1 August 2003). "Generics - equal or not?". Australian Prescriber. 26 (4): 85–87. doi:10.18773/austprescr.2003.063.
- ^ WHO Guidance for organizations performing in vivo bioequivalence studies (PDF). WHO Technical Report Series No. 996, Annex 9 (Report). World Health Organization. 2016.
- ^ Center for Drug Evaluation and Research (2003). "Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products – General Considerations" (PDF). United States Food and Drug Administration.