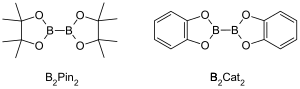
Metal-catalyzed C–H borylation reactions are transition metal catalyzed organic reactions that produce an organoboron compound through functionalization of aliphatic and aromatic C–H bonds and are therefore useful reactions for carbon–hydrogen bond activation.[1] Metal-catalyzed C–H borylation reactions utilize transition metals to directly convert a C–H bond into a C–B bond. This route can be advantageous compared to traditional borylation reactions by making use of cheap and abundant hydrocarbon starting material, limiting prefunctionalized organic compounds, reducing toxic byproducts, and streamlining the synthesis of biologically important molecules.[2][3] Boronic acids, and boronic esters are common boryl groups incorporated into organic molecules through borylation reactions.[4] Boronic acids are trivalent boron-containing organic compounds that possess one alkyl substituent and two hydroxyl groups. Similarly, boronic esters possess one alkyl substituent and two ester groups. Boronic acids and esters are classified depending on the type of carbon group (R) directly bonded to boron, for example alkyl-, alkenyl-, alkynyl-, and aryl-boronic esters. The most common type of starting materials that incorporate boronic esters into organic compounds for transition metal catalyzed borylation reactions have the general formula (RO)2B-B(OR)2. For example, bis(pinacolato)diboron (B2Pin2), and bis(catecholato)diborane (B2Cat2) are common boron sources of this general formula.[5]
The boron atom of a boronic ester or acid is sp2 hybridized possessing a vacant p orbital, enabling these groups to act as Lewis acids. The C–B bond of boronic acids and esters are slightly longer than typical C–C single bonds with a range of 1.55-1.59 Å. The lengthened C–B bond relative to the C–C bond results in a bond energy that is also slightly less than that of C–C bonds (323 kJ/mol for C–B vs 358 kJ/mol for C–C).[6] The carbon–hydrogen bond has a bond length of about 1.09 Å, and a bond energy of about 413 kJ/mol. The C–B bond is therefore a useful intermediate as a bond that replaces a typically unreactive C–H bond.
Organoboron compounds are organic compounds containing a carbon-boron bond. Organoboron compounds have broad applications for chemical synthesis because the C–B bond can easily be converted into a C–X (X = Br, Cl), C–O, C–N, or C–C bond. Because of the versatility of the C–B bond numerous processes have been developed to incorporate them into organic compounds.[7] Organoboron compounds are traditionally synthesized from Grignard reagents through hydroboration, or diboration reactions.[8] Borylation provides an alternative.
- ^ Hartwig, John F. (2012). "Borylation and Silylation of C–H Bonds: A Platform for Diverse C–H Bond Functionalizations". Accounts of Chemical Research. 45 (6): 864–873. doi:10.1021/ar200206a. ISSN 0001-4842. PMID 22075137.
- ^ Cho, J. Y.; Tse, M. K.; Holmes, D.; Maleczka, R. E. Jr.; Smith, M. R. (2001). "Remarkably Selective Iridium Catalysts for the Elaboration of Aromatic C-H Bonds". Science. 295 (5553): 305–8. doi:10.1126/science.1067074. PMID 11719693. S2CID 21096755.
- ^ Ishiyama, T.; Nobuta, Y.; Hartwig, J. F.; Miyaura, N. Chem. Commun. 2003, 2924.
- ^ Brown, H. C.; Kramer, G. W.; Levy, A. B.; Midland, M. M. Organic Synthesis via Boranes; Wiley-Interscience: New York, 1975; Vol. 1.
- ^ Braunschweig, H.; Guethlein, F. (2011). "Transition-Metal-Catalyzed Synthesis of Diboranes(4)". Angewandte Chemie International Edition. 50 (52): 12613–12616. doi:10.1002/anie.201104854. PMID 22057739.
- ^ Hall, D. G. (2011) Structure, Properties, and Preparation of Boronic Acid Derivatives, in Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials (Volume 1 and 2), Second Edition (ed D. G. Hall), Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany. doi:10.1002/9783527639328.ch1
- ^ Mkhalid, Ibraheem A. I.; Barnard, Jonathan H.; Marder, Todd B.; Murphy, Jaclyn M.; Hartwig, John F. (2010). "C–H Activation for the Construction of C–B Bonds". Chemical Reviews. 110 (2): 890–931. doi:10.1021/cr900206p. PMID 20028025.
- ^ Wade, L. G., Organic Chemistry. Upper Saddle River: Pearson Education, Inc., 2010.