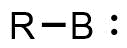
A borylene is the boron analogue of a carbene.[1][2][3][4] The general structure is R-B: with R an organic moiety and B a boron atom with two unshared electrons. Borylenes are of academic interest in organoboron chemistry. A singlet ground state is predominant with boron having two vacant sp2 orbitals and one doubly occupied one. With just one additional substituent the boron is more electron deficient than the carbon atom in a carbene. For this reason stable borylenes are more uncommon than stable carbenes. Some borylenes such as boron monofluoride (BF) and boron monohydride (BH) the parent compound also known simply as borylene, have been detected in microwave spectroscopy and may exist in stars. Other borylenes exist as reactive intermediates and can only be inferred by chemical trapping.
The first stable terminal borylene complex [(OC)5WBN(SiMe3)2] was reported by Holger Braunschweig et al. in 1998.[5][6] In this compound a borylene is coordinated to a transition metal. Borylenes are also stabilized as Lewis base adducts, e.g. with a NHC carbene.[7] Other strategies are the use of cyclic alkyl amino carbenes (CAACs)[8] and other Lewis bases,[9] and their use as bis-adducts.[10]
- ^ Cite error: The named reference
Braunschweigwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Bertrand2017was invoked but never defined (see the help page). - ^ Cite error: The named reference
Braunschweig2013was invoked but never defined (see the help page). - ^ Braunschweig, Holger; Dewhurst, Rian D.; Schneider, Achim (2010-07-14). "Electron-Precise Coordination Modes of Boron-Centered Ligands". Chemical Reviews. 110 (7): 3924–3957. doi:10.1021/cr900333n. ISSN 0009-2665. PMID 20235583.
- ^ Cite error: The named reference
braunschweig1998was invoked but never defined (see the help page). - ^ Cite error: The named reference
Braunschweig2015was invoked but never defined (see the help page). - ^ Cite error: The named reference
wangwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Dahchehwas invoked but never defined (see the help page). - ^ Braunschweig, Holger; Dewhurst, Rian D.; Hupp, Florian; Nutz, Marco; Radacki, Krzysztof; Tate, Christopher W.; Vargas, Alfredo (2015). "Multiple complexation of CO and related ligands to a main-group element". Nature. 522 (7556): 327–330. Bibcode:2015Natur.522..327B. doi:10.1038/nature14489. PMID 26085273. S2CID 4454142.
- ^ Cite error: The named reference
kinjowas invoked but never defined (see the help page).