 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /bjuːˈpɪvəkeɪn/ |
| Trade names | Marcaine, Sensorcaine, Posimir, others |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Parenteral, topical, implant |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Protein binding | 95% |
| Metabolism | Liver |
| Onset of action | Within 15 min[5] |
| Elimination half-life | 3.1 hours (adults)[5] 8.1 hours (neonates)[5] |
| Duration of action | 2 to 8 hr[6] |
| Excretion | Kidney, 4–10% |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.993 |
| Chemical and physical data | |
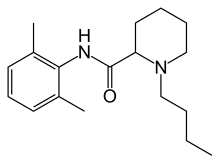
| Formula | C18H28N2O |
| Molar mass | 288.435 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 107 to 108 °C (225 to 226 °F) |
| |
| |
| | |
Bupivacaine, marketed under the brand name Marcaine among others, is a medication used to decrease sensation in a specific small area.[5] In nerve blocks, it is injected around a nerve that supplies the area, or into the spinal canal's epidural space.[5] It is available mixed with a small amount of epinephrine to increase the duration of its action.[5] It typically begins working within 15 minutes and lasts for 2 to 8 hours.[5][6]
Possible side effects include sleepiness, muscle twitching, ringing in the ears, changes in vision, low blood pressure, and an irregular heart rate.[5] Concerns exist that injecting it into a joint can cause problems with the cartilage.[5] Concentrated bupivacaine is not recommended for epidural freezing.[5] Epidural freezing may also increase the length of labor.[5] It is a local anaesthetic of the amide group.[5]
Bupivacaine was discovered in 1957.[7] It is on the World Health Organization's List of Essential Medicines.[8] Bupivacaine is available as a generic medication.[5][9] An implantable formulation of bupivacaine (Xaracoll) was approved for medical use in the United States in August 2020.[10][11][12]
- ^ "Bupivacaine Use During Pregnancy". Drugs.com. 13 April 2020. Retrieved 21 September 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Marcaine- bupivacaine hydrochloride injection, solution Marcaine with epinephrine- bupivacaine hydrochloride and epinephrine bitartrate injection, solution". DailyMed. Retrieved 13 February 2021.
- ^ "Sensorcaine MPF- bupivacaine hydrochloride injection, solution". DailyMed. Retrieved 13 February 2021.
- ^ a b c d e f g h i j k l m "Bupivacaine Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 30 June 2015. Retrieved 1 August 2015.
- ^ a b Whimster DS (1997). Cambridge textbook of accident and emergency medicine. Cambridge: Cambridge University Press. p. 194. ISBN 9780521433792. Archived from the original on 5 October 2015.
- ^ Egan TD (2013). Pharmacology and physiology for anesthesia : foundations and clinical application. Philadelphia, PA: Elsevier/Saunders. p. 291. ISBN 9781437716795. Archived from the original on 12 May 2016.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ Hamilton R (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 22. ISBN 9781284057560.
- ^ "Xaracoll: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 2 September 2020.
- ^ Cite error: The named reference
FDA Xaracoll approval letterwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Innocoll PRwas invoked but never defined (see the help page).