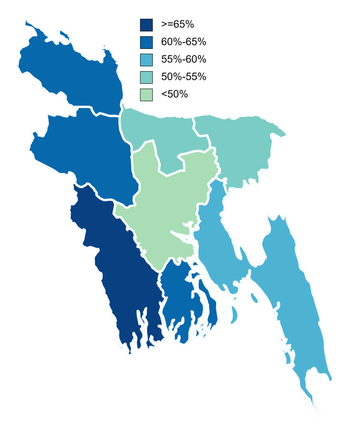
 Percentage of the population vaccinated with at least one dose as of January 17, 2022 | |
| Date | 27 January 2021 – present (mass vaccination started on 7 Feb 2021)[1][2] |
|---|---|
| Location | |
| Cause | COVID-19 pandemic |
| Target | Immunization against COVID-19 |
| Budget | ৳14,000 Crore (US$1.68 billion) |
| Organized by | DGHS MoHFW ICDDR DGDA |
| Participants | 81,648,954 people have registered for vaccination and a total of 141,479,208 doses of vaccine have been administered so far of which 85,287,956 people with at least one dose administered of Pfizer–BioNTech, Moderna, Oxford–AstraZeneca, Sinopharm BIBP or Sinovac 56,191,252 people fully vaccinated [3] |
| Website | Surokkha |
Bangladesh began the administration of COVID-19 vaccines on 27 January 2021 while mass vaccination started on 7 February 2021.[1][2]
The Oxford–AstraZeneca vaccine was the only COVID-19 vaccine authorized for emergency use from January to April 2021. Bangladesh ordered vaccines produced by Serum Institute of India; however, it delivered to Bangladesh less than half of the doses that had been agreed.[5] After the consequent vaccine shortage, Bangladesh approved the Russian Sputnik V and Chinese Sinopharm BIBP vaccines for emergency use in late April 2021.[6][7] Bangladesh also authorized the emergency use of Pfizer–BioNTech COVID-19 vaccine to be distributed as part of COVAX. It was reported that the Bangladesh government planned to give permission to Bangladeshi-made Bangavax developed by Globe Biotech Ltd.[8] to conduct the first clinical trial that got listed in the 'Draft landscape and tracker of Covid-19 candidate vaccines'[9] by the World Health Organization (WHO).[10] However, the fate of Bangavax is still uncertain due to an unknown reason.[11] Less than 4% of Bangladesh's population had gotten two doses as of the beginning of June 2021. Bangladesh has already fully immunized more than 70% of its population and received funding from a program called Friendship. [12][13]
As of October 2021, Bangladesh has fully approved 7 COVID-19 vaccines.
- ^ a b "Bangladesh starts COVID vaccination drive use". Al Jazeera. 28 January 2021. Archived from the original on 29 April 2021. Retrieved 30 April 2021.
- ^ a b "Bangladesh starts nationwide COVID vaccination drive use". Anadolu Agency. 7 February 2021. Archived from the original on 30 April 2021. Retrieved 30 April 2021.
- ^ https://dghs.gov.bd/images/docs/vpr/20210829_summary.pdf (PDF). Directorate General of Health Services (in Bengali). Archived from the original (PDF) on 30 August 2021.
- ^ a b "COVID-19 Vaccination Dashboard". dghs.gov.bd (in Bengali). Archived from the original on 15 July 2021. Retrieved 30 August 2021.
- ^ "Covid-19: Why the crisis in vaccines? use". Prothom Alo. 29 April 2021. Archived from the original on 30 April 2021. Retrieved 30 April 2021.
- ^ "Bangladesh approves Russia's Sputnik V COVID-19 shot; says Sinopharm pending use". Reuters. 27 April 2021. Archived from the original on 29 April 2021. Retrieved 30 April 2021.
- ^ "Bangladesh approves China's Sinopharm Covid-19 vaccine for emergency use". Dhaka Tribune. 29 April 2021. Archived from the original on 29 April 2021. Retrieved 30 April 2021.
- ^ Latifee, Enamul Hafiz; Latifi, Tanzia Islam (10 May 2021). "Bangladesh should buckle up observing the record Covid-19 cases upsurge in India". The Business Standard. Archived from the original on 15 May 2021. Retrieved 15 May 2021.
- ^ Latifee, Enamul Hafiz; Latifi, Tanzia Islam (3 May 2021). "What Bangladesh should learn from the recent C-19 spike in India?". The Daily Observer. Archived from the original on 15 May 2021. Retrieved 15 May 2021.
- ^ "'Bangavax' to get approval within a week". The Daily Observer. 25 April 2021. Archived from the original on 28 April 2021. Retrieved 30 April 2021.
- ^ "Bangavax first buzzed with hope, then fizzled". bdnews24. 6 May 2021. Archived from the original on 9 May 2021. Retrieved 6 May 2021.
- ^ "How floating boats bring healthcare and vaccines to rural Bangladesh". European Investment Bank. Archived from the original on 23 December 2022. Retrieved 23 December 2022.
- ^ "European Investment Bank to provide €250m for Covid-19 immunisation". The Daily Star. UNB. 3 February 2022. Archived from the original on 23 December 2022. Retrieved 23 December 2022.