 | |
| Clinical data | |
|---|---|
| Trade names | Cometriq, Cabometyx, others |
| Other names | XL184, BMS907351, cabozantinib s-malate |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613015 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | ≥99.7% |
| Metabolism | Liver (CYP3A4-mediated) |
| Elimination half-life | 110 hours |
| Excretion | Feces (54%), urine (27%) |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.221.147 |
| Chemical and physical data | |
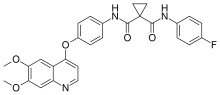
| Formula | C28H24FN3O5 |
| Molar mass | 501.514 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cabozantinib, sold under the brand names Cometriq and Cabometyx among others, is an anti-cancer medication used to treat medullary thyroid cancer, renal cell carcinoma, and hepatocellular carcinoma.[9][10] It is a small-molecule tyrosine-kinase inhibitor (TKI) of c-Met (HGFR) and VEGFR2, and also inhibits AXL, RET, and FLT3.[8][7] It was discovered and developed by Exelixis Inc.
In November 2012, cabozantinib in its capsule formulation was approved by the US Food and Drug Administration (FDA) under the name Cometriq for treating people with medullary thyroid cancer.[13][14] The capsule form was approved in the European Union for the same purpose in 2014.[11] In April 2016, the FDA granted approval for marketing the tablet formulation (Cabometyx) as a second line treatment for kidney cancer[15][16] and the same was approved in the European Union in September of that year.[12] The brands Cometriq and Cabometyx have different formulations and are not interchangeable.[17]
- ^ "Cabozantinib Use During Pregnancy". Drugs.com. 30 March 2020. Retrieved 23 September 2020.
- ^ "Cabometyx (Ipsen Pty Ltd)". Therapeutic Goods Administration (TGA). 13 January 2023. Retrieved 9 April 2023.
- ^ "Cabometyx cabozantinib (as (S)-malate) 20 mg film-coated tablet bottle (283800)". Therapeutic Goods Administration (TGA). 27 May 2022. Retrieved 9 April 2023.
- ^ "AusPAR: Cabozantinib". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 9 April 2023.
- ^ "Summary Basis of Decision (SBD) for Cabometyx". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- ^ "Drug and medical device highlights 2018: Helping you maintain and improve your health". Health Canada. 14 October 2020. Retrieved 17 April 2024.
- ^ a b Cite error: The named reference
UKlabelTabletwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
UKlabelCapsulewas invoked but never defined (see the help page). - ^ a b "Cabometyx- cabozantinib tablet". DailyMed. 21 July 2020. Retrieved 23 September 2020.
- ^ a b "Cometriq- cabozantinib kit Cometriq- cabozantinib capsule". DailyMed. 11 February 2020. Retrieved 23 September 2020.
- ^ a b Cite error: The named reference
Cometriq EPARwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Cabometyx EPARwas invoked but never defined (see the help page). - ^ Cite error: The named reference
FDA2012was invoked but never defined (see the help page). - ^ Cite error: The named reference
Cometriq FDA approvalwas invoked but never defined (see the help page). - ^ Cite error: The named reference
FDA2016-04was invoked but never defined (see the help page). - ^ Cite error: The named reference
Cabometyx FDA approvalwas invoked but never defined (see the help page). - ^ Cite error: The named reference
FDA Cabometyx 20171219was invoked but never defined (see the help page).