 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Daivonex, Dovonex, Sorilux |
| Other names | calcipotriene (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608018 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Topical administration |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 5 to 6% |
| Metabolism | Liver |
| Excretion | Biliary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.119.473 |
| Chemical and physical data | |
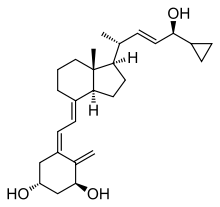
| Formula | C27H40O3 |
| Molar mass | 412.614 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Calcipotriol, also known as calcipotriene, is a synthetic derivative of calcitriol, a form of vitamin D. It is used in the treatment of psoriasis.[1] It is safe for long-term application in psoriatic skin conditions.[medical citation needed]
It was patented in 1985 and approved for medical use in 1991.[2] It is marketed under the trade name "Dovonex" in the United States, "Daivonex" outside North America, and "Psorcutan" in Germany.[citation needed]
It is on the World Health Organization's List of Essential Medicines.[3]
Calcipotriol is also available as Calcipotriol/betamethasone dipropionate, a fixed-dose combination medication with the synthetic corticosteroid betamethasone dipropionate for the treatment of plaque psoriasis.[4]
- ^ Prendergast B, Harrison J, Kulkarni K, Baguneid M (2016-10-27). Oxford Handbook of Key Clinical Evidence. Oxford University Press. p. 101. ISBN 9780198729426.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 452. ISBN 9783527607495.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "Taclonex- calcipotriene and betamethasone dipropionate ointment". DailyMed. 21 May 2020. Retrieved 19 October 2020.