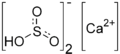
| |

| |
| Names | |
|---|---|
| IUPAC name
Calcium hydrogen sulfite
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.034.007 |
| E number | E227 (preservatives) |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Ca(HSO3)2 | |
| Molar mass | 202.22 g/mol |
| Melting point | 203 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Calcium bisulfite (calcium bisulphite or calcium hydrogen sulfite) is an inorganic compound which is the salt of a calcium cation and a bisulfite anion. It may be prepared by treating lime with an excess of sulfur dioxide and water. As a food additive it is used as a preservative under the E number E227. Calcium bisulfite is an acid salt and behaves like an acid in aqueous solution. It is used in the sulfite process for producing paper from wood chips.[1]
- ^ Patt, Rudolf; Kordsachia, Othar; Süttinger, Richard; Ohtani, Yoshito; Hoesch, Jochen F.; Ehrler, Peter; Eichinger, Rudolf; Holik, Herbert; Hamm, Udo; Rohmann, Michael E.; Mummenhoff, Peter; Petermann, Erich; Miller, Richard F.; Frank, Dieter; Wilken, Renke; Baumgarten, Heinrich L.; Rentrop, Gert-Heinz (2000). "Paper and Pulp". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a18_545. ISBN 3527306730.