
| |
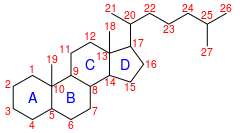 IUPAC numbering[1]
| |
| Names | |
|---|---|
| IUPAC name
Cholestane
| |
| Systematic IUPAC name
(1R,3aS,3bR,9aS,9bS,11aR)-9a,11a-Dimethyl-1-[(2R)-6-methylheptan-2-yl]hexadecahydro-1H-cyclopenta[a]phenanthrene | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.035.496 |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C27H48 | |
| Molar mass | 372.681 g·mol−1 |
| Density | 0.911 g/ml |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cholestane is a saturated tetracyclic triterpene. This 27-carbon biomarker is produced by diagenesis of cholesterol and is one of the most abundant biomarkers in the rock record.[2] Presence of cholestane, its derivatives and related chemical compounds in environmental samples is commonly interpreted as an indicator of animal life and/or traces of O2, as animals are known for exclusively producing cholesterol, and thus has been used to draw evolutionary relationships between ancient organisms of unknown phylogenetic origin and modern metazoan taxa.[3] Cholesterol is made in low abundance by other organisms (e.g., rhodophytes, land plants), but because these other organisms produce a variety of sterols it cannot be used as a conclusive indicator of any one taxon.[4][5] It is often found in analysis of organic compounds in petroleum.
- ^ The Nomenclature of Steroids Archived 2011-05-14 at the Wayback Machine, IUPAC
- ^ Peters, Kenneth E. (Kenneth Eric), 1950- (2007). The biomarker guide. Cambridge University Press. ISBN 9780521039987. OCLC 1015511618.
{{cite book}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Bobrovskiy, Ilya; Hope, Janet M.; Ivantsov, Andrey; Nettersheim, Benjamin J.; Hallmann, Christian; Brocks, Jochen J. (2018-09-20). "Ancient steroids establish the Ediacaran fossil Dickinsonia as one of the earliest animals". Science. 361 (6408): 1246–1249. Bibcode:2018Sci...361.1246B. doi:10.1126/science.aat7228. hdl:1885/230014. ISSN 0036-8075. PMID 30237355.
- ^ Combaut, Georges; Saenger, Peter (April 1984). "Sterols of the amansieae (rhodomelaceae: Rhodophyta)". Phytochemistry. 23 (4): 781–782. Bibcode:1984PChem..23..781C. doi:10.1016/s0031-9422(00)85025-6. ISSN 0031-9422.
- ^ Sonawane, Prashant D.; Pollier, Jacob; Panda, Sayantan; Szymanski, Jedrzej; Massalha, Hassan; Yona, Meital; Unger, Tamar; Malitsky, Sergey; Arendt, Philipp; Pauwels, Laurens; Almekias-Siegl, Efrat (2016-12-22). "Plant cholesterol biosynthetic pathway overlaps with phytosterol metabolism". Nature Plants. 3 (1): 16205. doi:10.1038/nplants.2016.205. ISSN 2055-0278. PMID 28005066. S2CID 5518449.