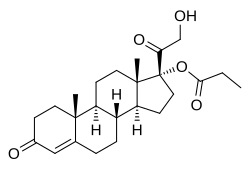
 | |
| Clinical data | |
|---|---|
| Trade names | Winlevi |
| Other names | CB-03-01; Breezula; 11-Deoxycortisol 17α-propionate; 17α-(Propionyloxy)- deoxycorticosterone; 21-Hydroxy-3,20-dioxopregn-4-en-17-yl propionate |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.810 |
| Chemical and physical data | |
| Formula | C24H34O5 |
| Molar mass | 402.531 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Clascoterone, sold under the brand name Winlevi, is an antiandrogen medication which is used topically in the treatment of acne.[5][6][7] It is also under development in a higher concentration for the treatment of androgen-dependent scalp hair loss, under the brand name Breezula.[6] The medication is used as a cream by application to the skin, for instance the face and scalp.[7]
Clascoterone is an antiandrogen, or antagonist of the androgen receptor (AR), the biological target of androgens such as testosterone and dihydrotestosterone.[8][9] It shows minimal systemic absorption when applied to skin.[7]
The medication, developed by Cassiopea and Intrepid Therapeutics,[6] was approved by the US Food and Drug Administration (FDA) for acne in August 2020.[10][11] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[12]
- ^ a b "Winlevi APMDS". Therapeutic Goods Administration (TGA). 17 May 2024. Retrieved 10 June 2024.
- ^ "Details for: Winlevi". Health Canada. 8 September 2023. Retrieved 3 March 2024.
- ^ "Summary Basis of Decision for Winlevi". Health Canada. 30 August 2023. Retrieved 4 October 2023.
- ^ "Details for: Winlevi". Health Canada. 8 September 2023. Retrieved 4 October 2023.
- ^ a b "Winlevi (clascoterone) cream, for topical use" (PDF). Cassiopea. Retrieved 9 September 2020.
- ^ a b c "Clascoterone - Cassiopea - AdisInsight".
- ^ a b c Kircik LH (July 2019). "What's new in the management of acne vulgaris". Cutis. 104 (1): 48–52. PMID 31487336.
- ^ Cite error: The named reference
pmid30811143was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid31141847was invoked but never defined (see the help page). - ^ "Cassiopea Receives FDA Approval for Winlevi (clascoterone cream 1%), First-in-Class Topical Acne Treatment Targeting the Androgen Receptor". Cassiopea (Press release). Archived from the original on 28 August 2020. Retrieved 30 August 2020.
- ^ "Winlevi: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 9 September 2020.
- ^ "New Drug Therapy Approvals 2020". U.S. Food and Drug Administration (FDA). 31 December 2020. Retrieved 17 January 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.