| Isomers of Cresol[1][2][3][4] | ||||
|---|---|---|---|---|
| Skeletal formula | 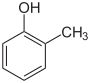 |
 |
||
| Ball-and-stick model | 
|

|
| |
| General | ||||
| Common name | o-cresol | m-cresol | p-cresol | |
| Preferred IUPAC name | 2-methylphenol | 3-methylphenol | 4-methylphenol | |
| Systematic name | 2-methylbenzenol | 3-methylbenzenol | 4-methylbenzenol | |
| Other names | ortho-cresol 2-hydroxytoluene |
meta-cresol 3-hydroxytoluene |
para-cresol 4-hydroxytoluene | |
| Molecular formula | C7H8O | |||
| SMILES | oc1c(C)cccc1 | oc1cc(C)ccc1 | oc1ccc(C)cc1 | |
| Molar mass | 108.14 g/mol | |||
| Appearance at room temperature and pressure |
colorless crystals | thicker liquid | greasy-looking solid | |
| CAS number | [95-48-7] | [108-39-4] | [106-44-5] | |
| mixture of cresols (tricresol): [1319-77-3] | ||||
| Properties | ||||
| Density and phase | 1.05 g/cm3, solid | 1.03 g/cm3, liquid | 1.02 g/cm3, liquid | |
| Solubility in pure water at 20−25 °C |
2.5 g/100 ml | 2.4 g/100 ml | 1.9 g/100 ml | |
| soluble in strongly alkaline water | ||||
| Melting point | 29.8 °C (303.0 K) | 11.8 °C (285.0 K) | 35.5 °C (309.7 K) | |
| Boiling point | 191.0 °C (464.2 K) | 202.0 °C (475.2 K) | 201.9 °C (475.1 K) | |
| Acidity (pKa) | 10.287 | 10.09 | 10.26 | |
| Viscosity | solid at 25 °C | ? cP at 25 °C | solid at 25 °C | |
| Structure | ||||
| Dipole moment | 1.35 D | 1.61 D | 1.58 D | |
| Hazards | ||||
| SDS | ||||
| Main hazards | flammable, ingestion and inhalation toxicity hazard | |||
| Flash point | 81 °C c.c. | 86 °C | 86 °C c.c. | |
| GHS pictograms |  
| |||
| RTECS number | GO6300000 | GO6125000 | GO6475000 | |
| Related compounds | ||||
| Related phenols | xylenols | |||
| Related compounds | bromocresol green, cresol red | |||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | ||||
Cresols (also known as hydroxytoluene, toluenol, benzol or cresylic acid) are a group of aromatic organic compounds. They are widely-occurring phenols (sometimes called phenolics) which may be either natural or manufactured. They are also categorized as methyl phenols. Cresols commonly occur as either solids or liquids because their melting points are generally close to room temperature. Like other types of phenols, they are slowly oxidized by exposure to air, and the resulting impurities often give the samples a yellow to brownish red tint. Cresols have an odor characteristic to that of other simple phenols, reminiscent to some of a "coal tar" smell. The name "cresol" is an adduct of phenol and their traditional source, creosote.
- ^ o-CRESOL (ICSC)
- ^ m-CRESOL (ICSC)
- ^ p-CRESOL (ICSC)
- ^ Pubchem. "o-cresol". pubchem.ncbi.nlm.nih.gov. Retrieved 2018-01-16.
