 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cytosar-U, Depocyt, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682222 |
| Pregnancy category |
|
| Routes of administration | injectable (intravenous injection or infusion, intrathecal, or subcutaneously) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 20% by mouth |
| Protein binding | 13% |
| Metabolism | Liver |
| Elimination half-life | biphasic: 10 min, 1–3 hr |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.188 |
| Chemical and physical data | |
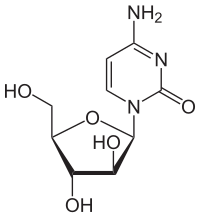
| Formula | C9H13N3O5 |
| Molar mass | 243.219 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cytarabine, also known as cytosine arabinoside (ara-C), is a chemotherapy medication used to treat acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), chronic myelogenous leukemia (CML), and non-Hodgkin's lymphoma.[2] It is given by injection into a vein, under the skin, or into the cerebrospinal fluid.[2] There is a liposomal formulation for which there is tentative evidence of better outcomes in lymphoma involving the meninges.[2]
Common side effects include bone marrow suppression, vomiting, diarrhea, liver problems, rash, ulcer formation in the mouth, and bleeding.[2] Other serious side effects include loss of consciousness, lung disease, and allergic reactions.[2] Use during pregnancy may harm the baby.[2] Cytarabine is in the antimetabolite and nucleoside analog families of medication.[3] It works by blocking the function of DNA polymerase.[2]
Cytarabine was patented in 1960 and approved for medical use in 1969.[4] It is on the World Health Organization's List of Essential Medicines.[5]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b c d e f g "Cytarabine". The American Society of Health-System Pharmacists. Archived from the original on 11 June 2016. Retrieved 8 December 2016.
- ^ British national formulary: BNF 69 (69 ed.). British Medical Association. 2015. p. 589. ISBN 9780857111562.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 511. ISBN 9783527607495. Archived from the original on 2016-12-20.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.