 | |
| Clinical data | |
|---|---|
| Pronunciation | /dəˈkɑːrbəˌziːn/ |
| Trade names | DTIC-Dome, others |
| Other names | DTIC[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682750 |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Metabolism | Extensive |
| Elimination half-life | 5 hours |
| Excretion | Kidney (40% as unchanged dacarbazine) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.022.179 |
| Chemical and physical data | |
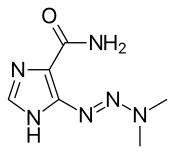
| Formula | C6H10N6O |
| Molar mass | 182.187 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Dacarbazine, also known as imidazole carboxamide and sold under the brand name DTIC-Dome, is a chemotherapy medication used in the treatment of melanoma and Hodgkin's lymphoma.[3] For Hodgkin's lymphoma it is often used together with vinblastine, bleomycin, and doxorubicin.[3] It is given by injection into a vein.[3]
Common side effects include loss of appetite, vomiting, low white blood cell count, and low platelets.[3] Other serious side effects include liver problems and allergic reactions.[3] It is unclear if use in pregnancy is safe for the baby.[3] Dacarbazine is in the alkylating agent and purine analog families of medication.[3]
Dacarbazine was approved for medical use in the United States in 1975.[3] It is on the World Health Organization's List of Essential Medicines.[4]
- ^ Elks J, Ganellin CR, eds. (1990). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 344–. doi:10.1007/978-1-4757-2085-3. ISBN 978-1-4757-2087-7.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved October 22, 2023.
- ^ a b c d e f g h "Dacarbazine". The American Society of Health-System Pharmacists. Archived from the original on September 11, 2017. Retrieved December 8, 2016.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.