 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Dantrium, Revonto, Ryanodex |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682576 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 70% |
| Metabolism | Liver |
| Excretion | Bile duct, kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.027.895 |
| Chemical and physical data | |
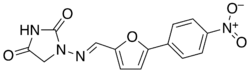
| Formula | C14H10N4O5 |
| Molar mass | 314.257 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |

Dantrolene sodium, sold under the brand name Dantrium among others, is a postsynaptic muscle relaxant that lessens excitation-contraction coupling in muscle cells.[5][6][7] It achieves this by inhibiting Ca2+ ions release from sarcoplasmic reticulum stores by antagonizing ryanodine receptors.[8] It is the primary drug used for the treatment and prevention of malignant hyperthermia, a rare, life-threatening disorder triggered by general anesthesia or drugs. It is also used in the management of neuroleptic malignant syndrome, muscle spasticity (e.g. after strokes, in paraplegia, cerebral palsy, or patients with multiple sclerosis), and poisoning by 2,4-dinitrophenol[9][10] or by the related compounds dinoseb and dinoterb.[11]
The most frequently occurring side effects include drowsiness, dizziness, weakness, general malaise, fatigue, and diarrhea.[5][6]
It is marketed by Par Pharmaceuticals LLC as Dantrium (in North America) and by Norgine BV as Dantrium, Dantamacrin, or Dantrolen (in Europe). A hospital is recommended to keep a minimum stock of 36 dantrolene vials totaling 720 mg, sufficient for a 70-kg person.[12]
- ^ "Dantrolene Use During Pregnancy". Drugs.com. 9 December 2019. Retrieved 6 July 2020.
- ^ "Product monograph brand safety updates". Health Canada. 6 June 2024. Retrieved 8 June 2024.
- ^ "Dantrium 25mg Capsules - Summary of Product Characteristics (SmPC)". (emc). 28 February 2020. Retrieved 6 July 2020.
- ^ Cite error: The named reference
Agilus EPARwas invoked but never defined (see the help page). - ^ a b "Dantrium- dantrolene sodium capsule". DailyMed. 1 February 2018. Retrieved 6 July 2020.
- ^ a b "Ryanodex dantrolene sodium- dantrolene sodium injection, suspension". DailyMed. 2 January 2019. Retrieved 6 July 2020.
- ^ "Revonto- dantrolene sodium injection, powder, lyophilized, for solution". DailyMed. 4 May 2020. Retrieved 6 July 2020.
- ^ Zucchi R, Ronca-Testoni S (March 1997). "The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states". Pharmacological Reviews. 49 (1): 1–51. PMID 9085308.
- ^ Kumar S, Barker K, Seger D (2002). "Dinitrophenol-Induced Hyperthermia Resolving With Dantrolene Administration. Abstracts of the North American Congress of Clinical Toxicology". Clin Toxicol. 40 (5): 599–673. doi:10.1081/clt-120016859. S2CID 218865517.
- ^ Barker K, Seger D, Kumar S (2006). "Comment on "Pediatric fatality following ingestion of Dinitrophenol: postmortem identification of a 'dietary supplement'"". Clinical Toxicology. 44 (3): 351. doi:10.1080/15563650600584709. PMID 16749560. S2CID 3057662.
- ^ Cite error: The named reference
Krausewas invoked but never defined (see the help page). - ^ Musselman ME, Saely S (January 2013). "Diagnosis and treatment of drug-induced hyperthermia". American Journal of Health-System Pharmacy. 70 (1): 34–42. doi:10.1186/1753-6561-9-S1-A32. PMC 4306034. PMID 23261898.