| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
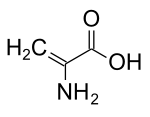
2-Aminoprop-2-enoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H5NO2 | |
| Molar mass | 87.08 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
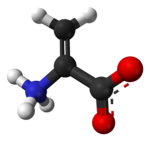
Dehydroalanine (Cα,β-didehydroalanine, α,β-di-dehydroalanine, 2-aminoacrylate, or 2,3-didehydroalanine) is a dehydroamino acid. It does not exist in its free form, but it occurs naturally as a residue found in peptides of microbial origin.[1] As an amino acid residue, it is unusual because it has an unsaturated backbone.[2]
- ^ Downs, DM; Ernst, DC (April 2015). "From microbiology to cancer biology: the Rid protein family prevents cellular damage caused by endogenously generated reactive nitrogen species". Molecular Microbiology. 96 (2): 211–9. doi:10.1111/mmi.12945. PMC 4974816. PMID 25620221.
- ^ Siodłak, Dawid (2015). "α,β-Dehydroamino Acids in Naturally Occurring Peptides". Amino Acids. 47 (1): 1–17. doi:10.1007/s00726-014-1846-4. PMC 4282715. PMID 25323736.