 | |
| Clinical data | |
|---|---|
| Other names | O-Desmethyltramadol; O-DSMT; Omnitram |
| Pharmacokinetic data | |
| Metabolism | CYP3A4 and CYP2B6[1] |
| Elimination half-life | 6-8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H23NO2 |
| Molar mass | 249.354 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
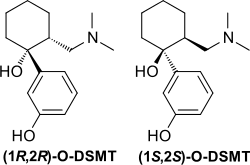
Desmetramadol (INN), also known as O-desmethyltramadol (O-DSMT), is an opioid analgesic and the main active metabolite of tramadol.[2] Tramadol is demethylated by the liver enzyme CYP2D6[3] to desmetramadol in the same way as codeine, and so similarly to the variation in effects seen with codeine, individuals who have a less active form of CYP2D6 will tend to have reduced analgesic effects from tramadol. Because desmetramadol itself does not need to be metabolized to induce an analgesic effect, it can be used in individuals with low CYP2D6 activity unlike tramadol.
- ^ Tramadol Pharmacokinetics, PharmGKB
- ^ Sevcik J, Nieber K, Driessen B, Illes P (September 1993). "Effects of the central analgesic tramadol and its main metabolite, O-desmethyltramadol, on rat locus coeruleus neurones". British Journal of Pharmacology. 110 (1): 169–76. doi:10.1111/j.1476-5381.1993.tb13788.x. PMC 2175982. PMID 8220877.
- ^ Borlak J, Hermann R, Erb K, Thum T (November 2003). "A rapid and simple CYP2D6 genotyping assay--case study with the analgetic tramadol". Metabolism. 52 (11): 1439–43. doi:10.1016/s0026-0495(03)00256-7. PMID 14624403.