 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
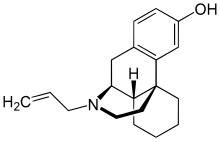
| Formula | C19H25NO |
| Molar mass | 283.415 g·mol−1 |
| 3D model (JSmol) | |
| |
Dextrallorphan (DXA) is a chemical of the morphinan class that is used in scientific research. It acts as a σ1 receptor agonist and NMDA receptor antagonist.[1][2][3][4] It has no significant affinity for the σ2, μ-opioid, or δ-opioid receptor, or for the serotonin or norepinephrine transporter.[2][5] As an NMDA receptor antagonist, in vivo, it is approximately twice as potent as dextromethorphan, and five-fold less potent than dextrorphan.[3]
- ^ Su TP (November 1982). "Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 223 (2): 284–90. PMID 6290634.
- ^ a b Codd EE, Shank RP, Schupsky JJ, Raffa RB (September 1995). "Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 274 (3): 1263–70. PMID 7562497.
- ^ a b Shukla VK, Lemaire S (January 1997). "N-methyl-D-aspartate antagonist activity of alpha- and beta-sulfallorphans" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 280 (1): 357–65. PMID 8996216.
- ^ Shannon HE (April 1983). "Pharmacological evaluation of N-allynormetazocine (SKF 10,047) on the basis of its discriminative stimulus properties in the rat". The Journal of Pharmacology and Experimental Therapeutics. 225 (1): 144–52. PMID 6834266.
- ^ He XS, Bowen WD, Lee KS, Williams W, Weinberger DR, de Costa BR (March 1993). "Synthesis and binding characteristics of potential SPECT imaging agents for sigma-1 and sigma-2 binding sites". Journal of Medicinal Chemistry. 36 (5): 566–71. doi:10.1021/jm00057a006. PMID 8496936.