 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Alone: Dinagest, Visanne, Zalkya With EV: Natazia, Qlaira With EE: Valette |
| Other names | DNG; Dienogestril; Cyanomethyldienolone; BAY 86-5258; Endometrion; M-18575; MJR-35; SH-660; SH-T00660AA; STS-557; ZK-37659; δ9-17α-Cyanomethyl-19-nortestosterone; 17α-Cyanomethylestra-4,9(10)-dien-17β-ol-3-one; 17β-Hydroxy-3-oxo-19-nor-17α-pregna-4,9-diene-21-nitrile |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | By mouth[1][2] |
| Drug class | Progestogen; Progestin; Steroidal antiandrogen |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 90%[3] |
| Protein binding | Albumin: 90%[3] Free: 10%[3] |
| Metabolism | Liver (reduction, hydroxylation via CYP3A4, removal of cyanomethyl group, conjugation)[3][5] |
| Metabolites | • 9α,10β-Dihydro-DNG[1] • 3,5α-Tetrahydro-DNG[1] (Both said to be inactive)[2][3] |
| Elimination half-life | 7.5–10.7 hours[2] |
| Excretion | Urine[4] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.167.087 |
| Chemical and physical data | |
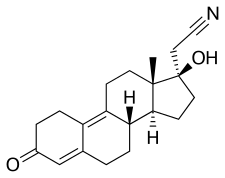
| Formula | C20H25NO2 |
| Molar mass | 311.425 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.2 g/cm3 |
| Melting point | 210 to 218 °C (410 to 424 °F) (experimental) |
| Boiling point | 549 °C (1,020 °F) |
| |
| (verify) | |
Dienogest, sold under the brand name Visanne among others, is a progestin medication which is used in birth control pills and in the treatment of endometriosis.[6][7][1][8][9][10] It is also used in menopausal hormone therapy and to treat heavy periods.[8][11][12] Dienogest is available both alone and in combination with estrogens.[13][11] It is taken by mouth.[1]
Side effects of dienogest include menstrual irregularities, headaches, nausea, breast tenderness, depression, and acne, among others.[14] Dienogest is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[1][2] It is a unique progestogen, with strong effects in the uterus.[2] The medication has some antiandrogenic activity, which may help to improve androgen-dependent symptoms like acne, and has no other important hormonal activity.[1][2][7][15][16]
Dienogest was discovered in 1979 and was introduced for medical use in 1995.[17][18][19] Additional formulations of dienogest were approved between 2007 and 2010.[10][20] It is sometimes referred to as a "fourth-generation" progestin.[21][22] Dienogest is marketed widely throughout the world.[13] It is available as a generic medication.[23]
- ^ a b c d e f g Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ^ a b c d e f Cite error: The named reference
pmid22364708was invoked but never defined (see the help page). - ^ a b c d e Bińkowska M, Woroń J (June 2015). "Progestogens in menopausal hormone therapy". Przeglad Menopauzalny = Menopause Review. 14 (2): 134–143. doi:10.5114/pm.2015.52154. PMC 4498031. PMID 26327902.
- ^ Bizzarri N, Remorgida V, Leone Roberti Maggiore U, Scala C, Tafi E, Ghirardi V, et al. (September 2014). "Dienogest in the treatment of endometriosis". Expert Opinion on Pharmacotherapy. 15 (13): 1889–1902. doi:10.1517/14656566.2014.943734. PMID 25069386. S2CID 37627607.
- ^ Stanczyk FZ (November 2003). "All progestins are not created equal". Steroids. 68 (10–13): 879–890. doi:10.1016/j.steroids.2003.08.003. PMID 14667980. S2CID 44601264.
- ^ "TGA eBS - Product and Consumer Medicine Information Licence".
- ^ a b Foster RH, Wilde MI (November 1998). "Dienogest". Drugs. 56 (5): 825–33, discussion 834–5. doi:10.2165/00003495-199856050-00007. PMID 9829156. S2CID 262326901.
- ^ a b "Dienogest - Bayer HealthCare Pharmaceuticals/Mochida Pharmaceutical - AdisInsight".
- ^ Stanczyk FZ, Bretsky (22 May 2013). "Biosynthesis, Transport, and Metabolism of Steroid Hormones". In Falcone T, Hurd WW (eds.). Clinical Reproductive Medicine and Surgery: A Practical Guide. Springer Science & Business Media. pp. 300–. ISBN 978-1-4614-6837-0.
Dienogest is a 19-nortestosterone derivative that is approved in the European Union for the treatment of endometriosis. It is not available in the United States as a separate drug. It is only available in the oral contraceptive Natazia (Bayer Pharmaceuticals, Montville, NJ, USA) (estradiol valerate/dienogest), which is a newer four-phasic pack that contains dienogest.
- ^ a b McCormack PL (November 2010). "Dienogest: a review of its use in the treatment of endometriosis". Drugs. 70 (16): 2073–2088. doi:10.2165/11206320-000000000-00000. PMID 20964453. S2CID 249871173.
- ^ a b Bartsch V, Römer T (2015). "Gynaecological uses of dienogest alone and in combination with oestrogens" (PDF). J Med Drug Rev. 5: 1–31. Archived from the original (PDF) on 2021-01-07. Retrieved 2018-01-08.
- ^ Cite error: The named reference
pmid23239397was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Drugs.comwas invoked but never defined (see the help page). - ^ "Dienogest". Australian Prescriber. 38 (4): 138–139. August 2015. doi:10.18773/austprescr.2015.050. PMC 4653971. PMID 26648643.
- ^ Raudrant D, Rabe T (2003). "Progestogens with antiandrogenic properties". Drugs. 63 (5): 463–492. doi:10.2165/00003495-200363050-00003. PMID 12600226. S2CID 28436828.
- ^ Regidor PA, Schindler AE (October 2017). "Antiandrogenic and antimineralocorticoid health benefits of COC containing newer progestogens: dienogest and drospirenone". Oncotarget. 8 (47): 83334–83342. doi:10.18632/oncotarget.19833. PMC 5669973. PMID 29137347.
- ^ Cite error: The named reference
MenzenbachHubner1984was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid10493599was invoked but never defined (see the help page). - ^ Cite error: The named reference
Jenapharmwas invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid21151673was invoked but never defined (see the help page). - ^ Hall S, Gebbie AE (11 July 2013). "Progestogens used in Contraceptives". In Briggs P, Kovacs G (eds.). Contraception: A Casebook from Menarche to Menopause. Cambridge University Press. pp. 52–. ISBN 978-1-107-43611-4.
- ^ Bitzer J (9 April 2015). "Progestogens in Contraception". In Carp HJ (ed.). Progestogens in Obstetrics and Gynecology. Springer. pp. 112–113, 170–. ISBN 978-3-319-14385-9.
- ^ "Generic Natazia Availability".