| |

| |
| Names | |
|---|---|
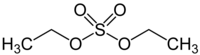
| Preferred IUPAC name
Diethyl sulfate | |
| Other names
Sulfuric acid diethyl ester
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.536 |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H10O4S | |
| Molar mass | 154.18 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.2 g/mL |
| Melting point | −25 °C (−13 °F; 248 K) |
| Boiling point | 209 °C (408 °F; 482 K) (decomposes) |
| decomposes in water | |
| Vapor pressure | 0.29 mm Hg |
| -86.8·10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H302, H312, H314, H332, H340, H350 | |
| P201, P202, P260, P261, P264, P270, P271, P280, P281, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P308+P313, P310, P312, P321, P322, P330, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 104 °C (219 °F; 377 K) |
| Related compounds | |
Related compounds
|
Dimethyl sulfate; diethyl sulfite |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diethyl sulfate (DES) is an organosulfur compound with the formula (C2H5)2SO4.[1][2] It occurs as a colorless, oily liquid with a faint peppermint odor. It is toxic, combustible, and likely carcinogenic chemical compound.[3][2] Diethyl sulfate is used as an ethylating agent.
- ^ Cite error: The named reference
Ullmannwas invoked but never defined (see the help page). - ^ a b "Diethyl Sulfate | CAMEO Chemicals | NOAA". cameochemicals.noaa.gov. Retrieved 2021-03-04.
- ^ "NCI Thesaurus". ncit.nci.nih.gov. Retrieved 2021-04-02.
